Are you looking for an answer to the topic “Which element has the electron configuration of 1s 2 2s 2 2p 6 3s 2 3p 4?“? We answer all your questions at the website Ecurrencythailand.com in category: +15 Marketing Blog Post Ideas And Topics For You. You will find the answer right below.
According to the periodic table, this element is sulfur.If you are referring to a neutral atom, then Vanadium (V) has that particular electron configuration.The electron configuration 1s22s22p63s23p2 is the element Silicon.
| Element | Atomic number | Electron configuration |
|---|---|---|
| silicon | 14 | 1s22s22p63s23p2 |
| phosphorus | 15 | 1s22s22p63s23p3 |
| sulfur | 16 | 1s22s22p63s23p4 |
| chlorine | 17 | 1s22s22p63s23p5 |
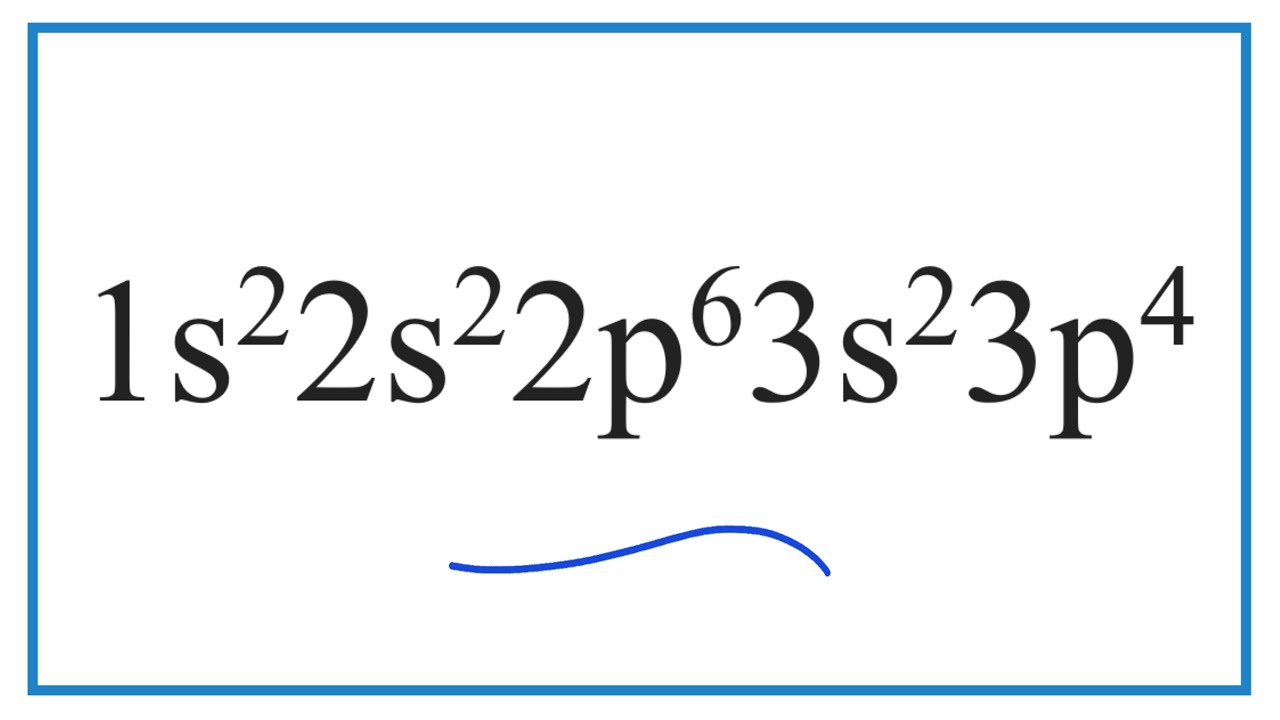
What element has an electron configuration of 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 and which block is it in?
If you are referring to a neutral atom, then Vanadium (V) has that particular electron configuration.
What atom matches this electron configuration 1s 2 2s 2 2p 6 3s 2?
The electron configuration 1s22s22p63s23p2 is the element Silicon.
Which element has the electron configuration of 1s2 2s2 2p6 3s2 3p4?
Images related to the topicWhich element has the electron configuration of 1s2 2s2 2p6 3s2 3p4?
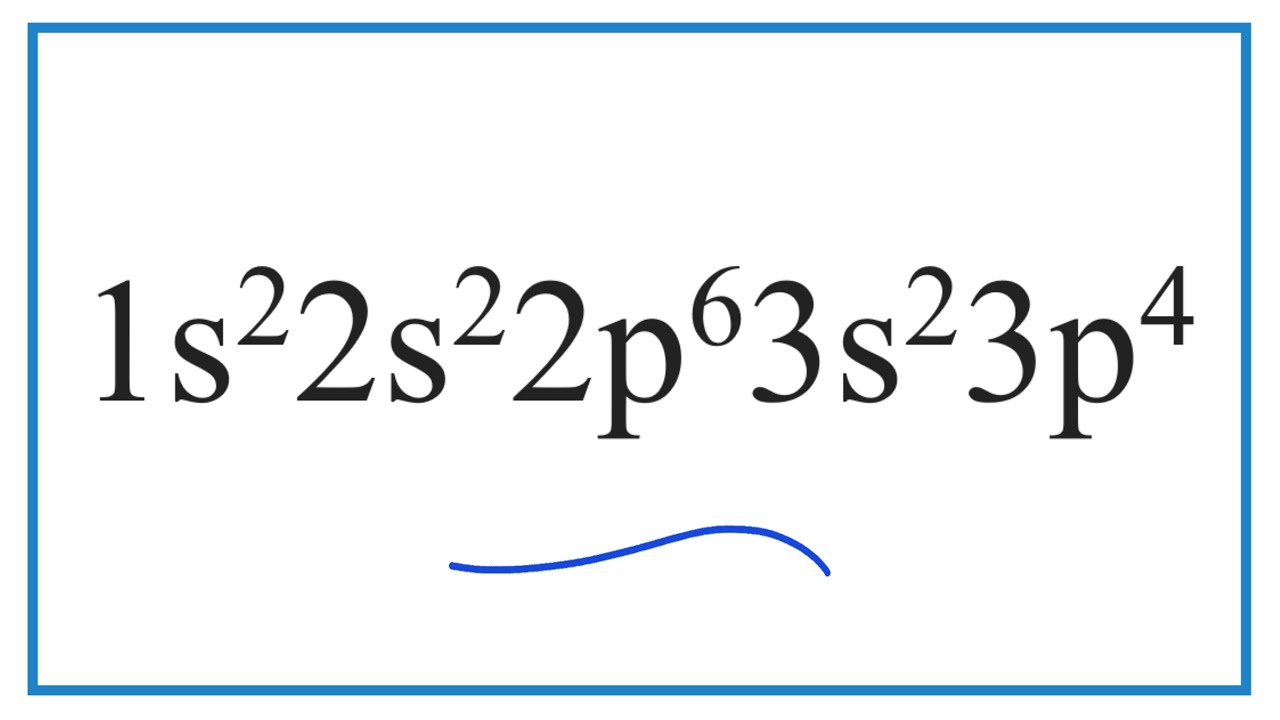
Which element has the electron configuration 1s 2 2s 2 2p 6 3s 2 3p 3?
| Element | Atomic number | Electron configuration |
|---|---|---|
| silicon | 14 | 1s22s22p63s23p2 |
| phosphorus | 15 | 1s22s22p63s23p3 |
| sulfur | 16 | 1s22s22p63s23p4 |
| chlorine | 17 | 1s22s22p63s23p5 |
What element is 1s2 2s2 2p6 3s2 3p6 4s2 3d7?
The electron configuration for Copper (Co) is: 1s2 2s2 2p6 3s3 3p6 4s2 3d7. A chemist would shorten this notation to just “3d7” – calling Copper by the subshell of highest energy that contains any electrons.
What is the atomic number of 1s2 2s2 2p6 3s2?
The element which present just below this element will have outermost electronic configuration as 4s24p3, so its full electronic configuration is 1s2,2s22p6,3s23p6,4s2, 3d10,4p3 and hence, its atomic number is 33.
What is the group and period of an element with an electron configuration of 1s2 2s2 2p6?
Expert-verified answer
The element with electron configuration 1s2 2s2 2p6 3s2 3p1 is Al as its total electron is 13. Al is a metal of group 13. It is a good conductor of heat and electricity. It resides in period 3 of the modern periodic table.
What element is 1s1?
| A | B |
|---|---|
| Hydrogen | 1s1 |
| Helium | 1s2 |
| Lithium | 1s2 2s1 |
| Boron | 1s2 2s2 2p1 |
See some more details on the topic Which element has the electron configuration of 1s 2 2s 2 2p 6 3s 2 3p 4? here:
What element has the electron configuration 1s^2 2s^2 2p^6 …
Z=16 , therefore the element is silicon. Explanation: The neutral atom contains as many electrons, negatively charged particles, …
The Electron Configurations of Atoms
magnesium, 12, 1s22s22p63s ; aluminum, 13, 1s22s22p63s23p ; silicon, 14, 1s22s22p63s23p ; phosphorus, 15, 1s22s22p63s23p …
What Element Has The Electron Configuration Of 1s2 2s2 2p6 …
Atomic no. of sulphur is 16 hence its electronic configuration is 2,8,6 [1s2,2s2,2p6,3s2,3p4] as it has 6 electrons in its outermost shell …
The Parts of the Periodic Table – Angelo State University
The electron configuration of an element is a list of the atomic orbitals which … Ra: 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 4f14 5s2 5p6 5d10 6s2 6p6 7s2.
How many electrons does Al contain if it has this kind of electron configuration 1s2 2s2 2p6 3s2 3p1?
Al has an atomic number of 13 . Thus , it has 13 electrons and 13 protons .
Which element has the electron configuration of? 1s2 2s2 2p6 3s2 3p6 4s2 ?
Images related to the topicWhich element has the electron configuration of? 1s2 2s2 2p6 3s2 3p6 4s2 ?
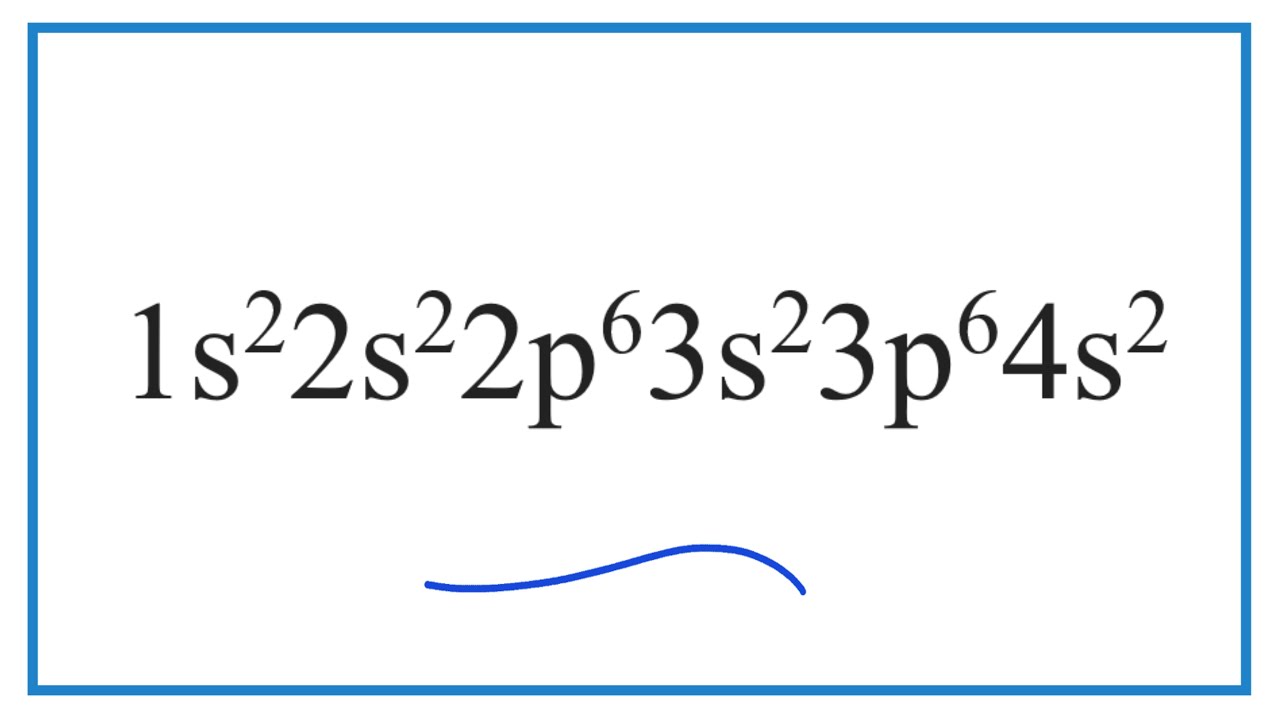
What element is Xe 6s2 4f14 5d9?
| Element Atomic Number | Element Symbol | Element Electron Configuration |
|---|---|---|
| 78 | Pt | [Xe] 4f14 5d9 6s1 |
| 79 | Au | [Xe] 4f14 5d10 6s1 |
| 80 | Hg | [Xe] 4f14 5d10 6s2 |
| 81 | Tl | [Xe] 4f14 5d10 6s2 6p1 |
What is 1s 2s 2p 3s 3p?
1s 2s 2p 3s 3p represents the electron orbital energy levels.
What is 1s 2s 2p in chemistry?
In order as: 1s, 2s, 2p, 3s, 3p, 4s, 3d, 4p, 5s, 4d, 5p… 1s will be filled first, with the maximum of 2 electrons. 2s will be filled next, with the maximum of 2 electrons. 2p will be filled next, with the maximum of 6 electrons.
What is 1s2 2s2 2p6?
b. 1s2 2s2 2p6 This represents 2 electrons in the s subshell of the first energy level , 2 electrons in the s subshell of the second energy level and 6 electrons in the p subshell of the second energy level.
What element is AR 4s2 3d7?
| A | B |
|---|---|
| [Ar] 4s2 3d7 | Ni |
| [Ne] 3s2 3p2 | Si |
| 1s2 2s2 2p6 3s1 | Na |
| 1s2 2s2 2p6 3s2 3p6 4s2 3d1 | Sc |
What is the atomic number of the element with 1s2 2s2 2p6 3s2 3p3 electronic configuration What will be the element below this element in the periodic table?
The electronic configuration 1s2,2s2,2p6,3s23p3 represents phosphorus with atomic number 15.
What is the group and period of an element with an electronic configuration of 1s2 2s2 2p6 Brainly?
Answer. Its position is : 2nd group 3rd period.
Which element has the electron configuration of 1s2 2s2 2p6 3s1?
Images related to the topicWhich element has the electron configuration of 1s2 2s2 2p6 3s1?

What is group 2 Period 3 on the periodic table?
…
Group 2A — The Alkaline Earth Metals.
| 3A | (13) |
|---|---|
| 4A | (14) |
| 5A | (15) |
| 6A | (16) |
| 7A | (17) |
How do you find the period and group of electron configuration?
- If the element is in s block, then the group number is equal to the number of valence electrons. …
- If the element is in the p block, then the number of the group can be determined by the formula: (number of valence electrons + 10).for groups.
Related searches to Which element has the electron configuration of 1s 2 2s 2 2p 6 3s 2 3p 4?
- 3s2 3p4 element
- 1s^2 2s^2 2p^6 3s^2 3p^1
- 1s2 2s2 2p6 3s2 3p1
- what element has this electron configuration 1s2 2s2 2p4
- 3s2 3p4 electron configuration
- 1s2s2p 3s3p electron configuration
- which element has the electron configuration of 1s 2 2s 2 2p 6 3s 2 3p 4
- 1s2 2s2 2p6 element
- 1s2 2s2 2p6 3s2 3p6 4s1 element
- 1s2 2s2 2p6 3s2 3p4 valence electrons
Information related to the topic Which element has the electron configuration of 1s 2 2s 2 2p 6 3s 2 3p 4?
Here are the search results of the thread Which element has the electron configuration of 1s 2 2s 2 2p 6 3s 2 3p 4? from Bing. You can read more if you want.
You have just come across an article on the topic Which element has the electron configuration of 1s 2 2s 2 2p 6 3s 2 3p 4?. If you found this article useful, please share it. Thank you very much.