Are you looking for an answer to the topic “Which factor increases the rate of a chemical reaction Brainly?“? We answer all your questions at the website Ecurrencythailand.com in category: +15 Marketing Blog Post Ideas And Topics For You. You will find the answer right below.
Keep Reading
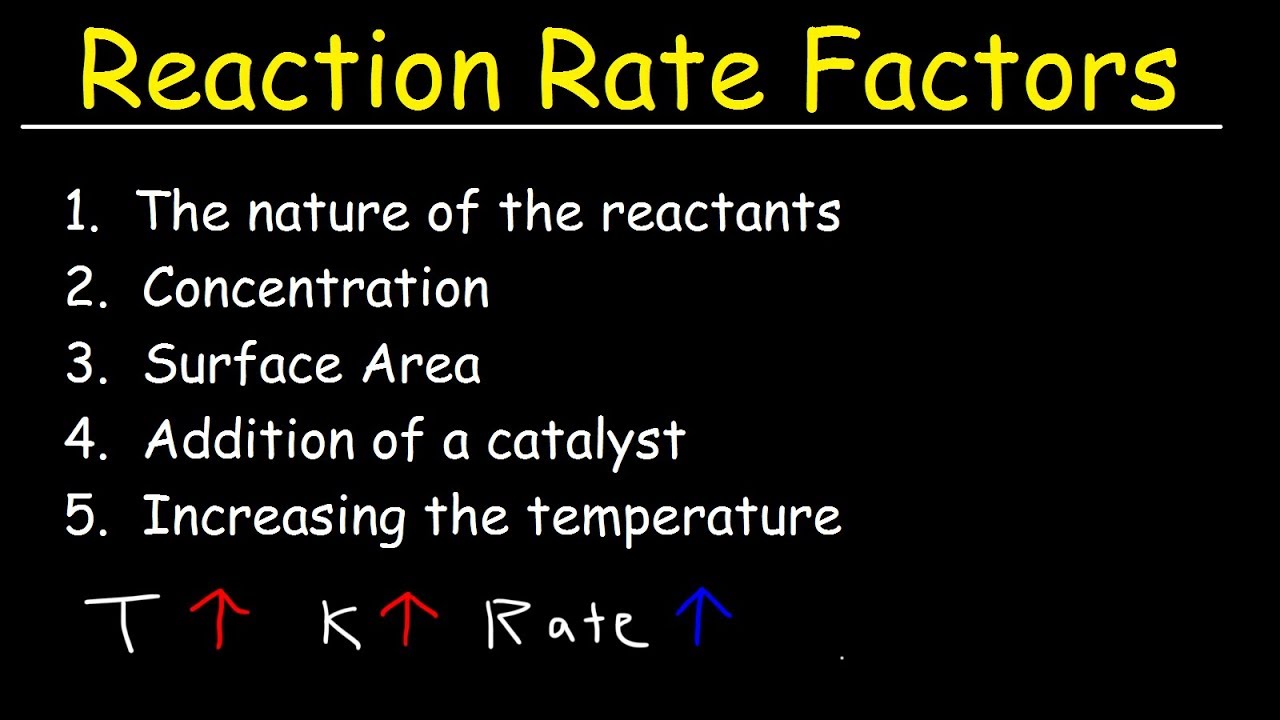
Which factors increases the rate of chemical reaction?
- Reactant concentration. Increasing the concentration of one or more reactants will often increase the rate of reaction. …
- Physical state of the reactants and surface area. …
- Temperature. …
- Presence of a catalyst.
What factors could be used to increase reaction rate Brainly?
Answer: increasing temperature– the particles of a molecule move faster and undergo more collision which leads to an increase in the speed of the reaction. -increasing pressure- means there is more particles of reactants in a reduced volume.
Factors Affecting the Rate of the Reaction – Chemical Kinetics
Images related to the topicFactors Affecting the Rate of the Reaction – Chemical Kinetics

Which factor increases the reaction rate by increasing particle speed?
Temperature Effects
As the average kinetic energy increases, the particles move faster and collide more frequently per unit time and possess greater energy when they collide. Both of these factors increase the reaction rate. Hence the reaction rate of virtually all reactions increases with increasing temperature.
Which three factors affect the rate of a chemical reaction Mcq?
- Heat of fusion.
- Heat of reaction.
- Free energy.
- Activation energy.
What factors increase the rate of a chemical reaction quizlet?
- Nature of Reactants.
- Surface Area (more = faster)
- Temperature (higher = faster)
- Concentration (larger = faster)
- Catalyst (present = faster)
Which factor does not increase the rate of a reaction?
The catalyst can be used for the effective increase of the reaction speed. But the increase of activation energy cannot increase the rate of a reaction. Only the lower activation energy can increase the reaction rate. Hence option e is correct.
What are the 5 factors that affect the rate of reaction?
Five factors typically affecting the rates of chemical reactions will be explored in this section: the chemical nature of the reacting substances, the state of subdivision (one large lump versus many small particles) of the reactants, the temperature of the reactants, the concentration of the reactants, and the …
See some more details on the topic Which factor increases the rate of a chemical reaction Brainly? here:
Which factor increases the rate of a chemical reaction? – Brainly.com
There are 3 factors which are; temperature, pressure and concentration. Still stuck? Get 1-on-1 help from an expert tutor now.
What factors could be used to increase reaction rate? Check …
The rate of a reaction can be increased by: -using a catalyst- it lowers the activation energy and leads to less energy required to break …
Write the factors of that affect the rate of the reaction – Brainly.in
1. temperature – increasing the temperature increases the rate of reaction because there are more collisions · 2. surface area – increasing …
12.2 Factors Affecting Reaction Rates – Chemistry – BC Open …
Activation energy is the minimum amount of energy required for a chemical reaction to proceed in the forward direction. A catalyst increases the reaction rate …
How catalyst increases the rate of reaction?
A catalyst is a substance that can be added to a reaction to increase the reaction rate without getting consumed in the process. Catalysts typically speed up a reaction by reducing the activation energy or changing the reaction mechanism. Enzymes are proteins that act as catalysts in biochemical reactions.
Which factors would increase the rate of a chemical reaction increasing the temperature removing products as they are formed adding a catalyst?
With an increase in temperature, there is an increase in the number of collisions. Increasing the concentration of a reactant increases the frequency of collisions between reactants and will, therefore, increase the reaction rate.
Which of the following factors decreases the rate of a chemical reaction?
The reaction rate decreases with a decrease in temperature. Catalysts can lower the activation energy and increase the reaction rate without being consumed in the reaction. Differences in the inherent structures of reactants can lead to differences in reaction rates.
GCSE Chemistry – Factors Affecting the Rate of Reaction #47
Images related to the topicGCSE Chemistry – Factors Affecting the Rate of Reaction #47

How can you speed up a chemical reaction?
- Use a Catalyst. A catalyst is a substance that can alter the rate of a chemical reaction. …
- Increase the Temperature. …
- Concentrate of the Reactants. …
- Increase the Surface Area of the Reactants.
Why does increasing the pressure of a gas increase the reaction rate?
Pressure. If the pressure of gaseous reactants is increased, there are more reactant particles for a given volume. There will be more collisions and so the reaction rate is increased. The higher the pressure of reactants, the faster the rate of a reaction will be.
What factors would affect the reaction rate Mcq?
- Increase the concentration of the reactants.
- Increase the surface area of the reactants.
- Increase the temperature of the reactants.
- Increase the volume that the reactants take up.
What is the rate of chemical reaction?
reaction rate, in chemistry, the speed at which a chemical reaction proceeds. It is often expressed in terms of either the concentration (amount per unit volume) of a product that is formed in a unit of time or the concentration of a reactant that is consumed in a unit of time.
Does reaction rate increase with temperature?
When the reactants are heated, the average kinetic energy of the molecules increases. This means that more molecules are moving faster and hitting each other with more energy. If more molecules hit each other with enough energy to react, then the rate of the reaction increases.
Which of the following actions would increase the rate of a reaction?
The reaction rate would increase. One way to increase the rate of a reaction occurring in solution is to stir the solution.
What are the 4 factors that affect a chemical reaction?
- surface area of a solid reactant.
- concentration or pressure of a reactant.
- temperature.
- nature of the reactants.
- presence/absence of a catalyst.
Which of the following factors will influence the rate of chemical reactions quizlet?
Five major factors influence reaction rate: structure of the reacting species, concentration of reactants, temperature of reactants, physical state of reactants, and *presence of a catalyst.
Factors affecting the rate of a chemical reaction WNOD4382
Images related to the topicFactors affecting the rate of a chemical reaction WNOD4382

Which of the following factors will increase the rate of reaction in the forward direction?
Decreasing the temperature will cause the reaction to proceed in the forward direction, increasing the equilibrium concentration of CO(g). 3. Increasing the amount of C(s) will cause the reaction to proceed in the forward direction, increasing the equilibrium concentration of CO(g).
Why increasing surface area increases the rate of reaction?
If the surface area of a reactant is increased: more particles are exposed to the other reactant. there is a greater chance of particles colliding, which leads to more successful collisions per second. the rate of reaction increases.
Related searches to Which factor increases the rate of a chemical reaction Brainly?
- what statement best describes what happens during the catalytic cycle?
- which component decreases the activation energy of a chemical reaction 3 points
- which component of a chemical reaction does not change from beginning to end? (3 points)
- which component decreases the activation energy of a chemical reaction
- which component is required for the activation of some enzymes
- which component decreases the activation energy of a chemical reaction? (3 points)
- what statement best describes what happens during the catalytic cycle
- which factor increases the rate of a chemical reaction quizlet
- which component decreases the activation energy of a chemical reaction?
- which component of a chemical reaction does not change from beginning to end 3 points
- what statement best describes what happens to a substrate after it bonds to an enzyme?
- what statement best describes what happens to a substrate after it bonds to an enzyme
- which component of a chemical reaction does not change from beginning to end
Information related to the topic Which factor increases the rate of a chemical reaction Brainly?
Here are the search results of the thread Which factor increases the rate of a chemical reaction Brainly? from Bing. You can read more if you want.
You have just come across an article on the topic Which factor increases the rate of a chemical reaction Brainly?. If you found this article useful, please share it. Thank you very much.