Are you looking for an answer to the topic “Which law of thermodynamics states that energy in a system Cannot be created or destroyed but can only be converted from one form to another?“? We answer all your questions at the website Ecurrencythailand.com in category: +15 Marketing Blog Post Ideas And Topics For You. You will find the answer right below.
The First Law of Thermodynamics (Conservation) states that energy is always conserved, it cannot be created or destroyed. In essence, energy can be converted from one form into another.The law of conservation of energy, also known as the first law of thermodynamics, states that the energy of a closed system must remain constant—it can neither increase nor decrease without interference from outside.The law of conservation of energy states that energy can neither be created nor destroyed – only converted from one form of energy to another. This means that a system always has the same amount of energy, unless it’s added from the outside.
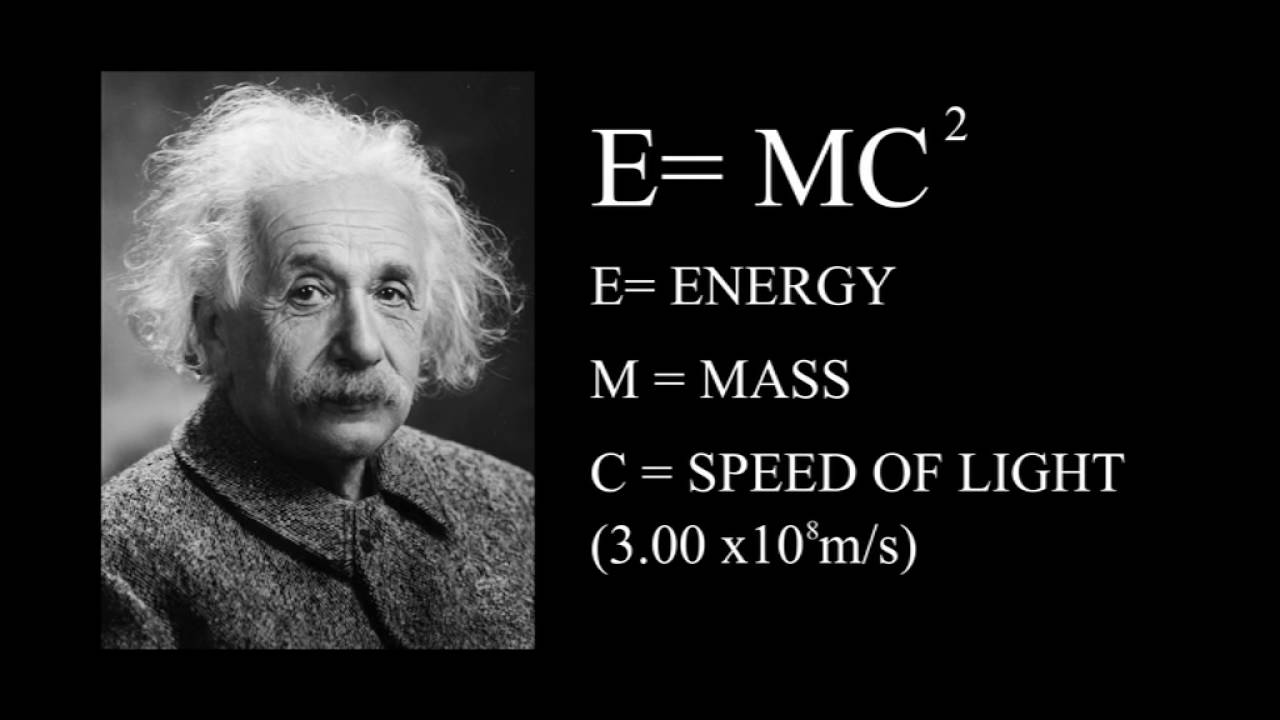
Which law of thermodynamics states energy Cannot be created or destroyed?
The law of conservation of energy, also known as the first law of thermodynamics, states that the energy of a closed system must remain constant—it can neither increase nor decrease without interference from outside.
What is the law that states that energy Cannot be created or destroyed only transformed from one energy to another?
The law of conservation of energy states that energy can neither be created nor destroyed – only converted from one form of energy to another. This means that a system always has the same amount of energy, unless it’s added from the outside.
1st Law Thermodynamics
Images related to the topic1st Law Thermodynamics
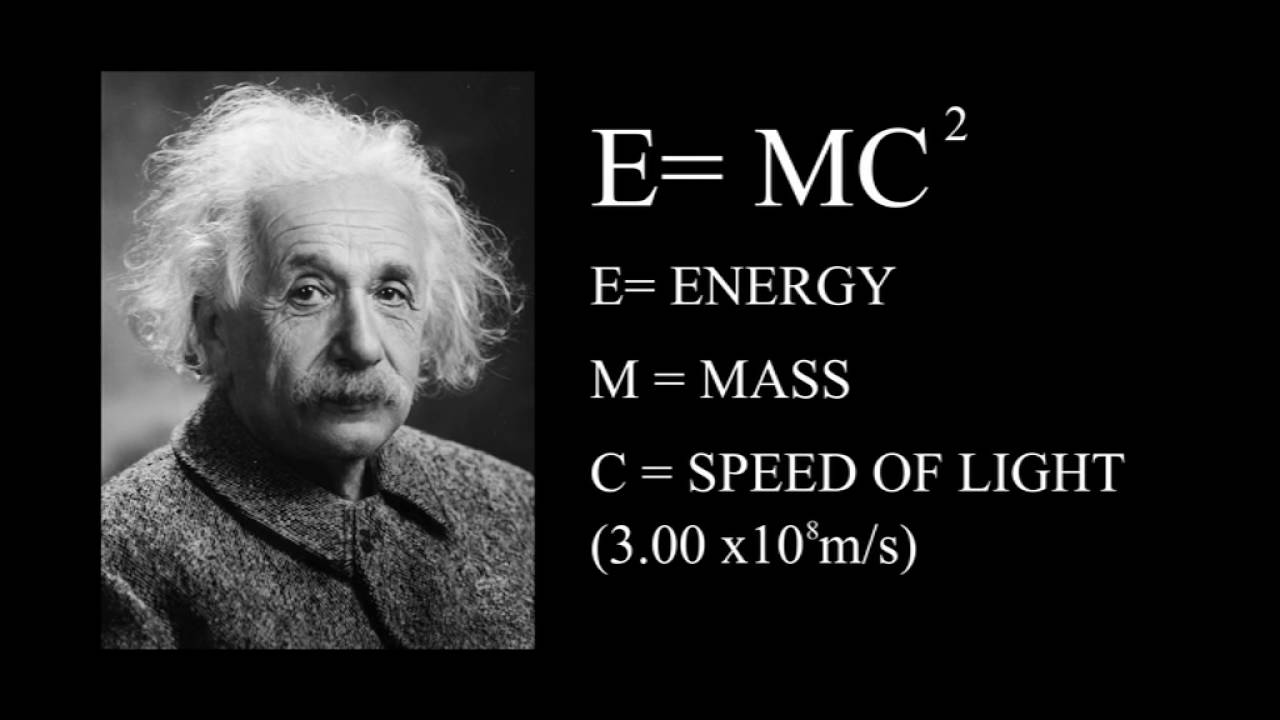
What are the 1st 2nd and 3rd laws of thermodynamics?
1st Law of Thermodynamics – Energy cannot be created or destroyed. 2nd Law of Thermodynamics – For a spontaneous process, the entropy of the universe increases. 3rd Law of Thermodynamics – A perfect crystal at zero Kelvin has zero entropy.
What does the 3rd law of thermodynamics state?
The third law of thermodynamics states that the entropy of a system approaches a constant value as the temperature approaches absolute zero. The entropy of a system at absolute zero is typically zero, and in all cases is determined only by the number of different ground states it has.
What does the 2nd law of thermodynamics state?
The Second Law of Thermodynamics states that “in all energy exchanges, if no energy enters or leaves the system, the potential energy of the state will always be less than that of the initial state.” This is also commonly referred to as entropy.
What is the 4 law of thermodynamics?
Fourth law of thermodynamics’: the dissipative component of evolution is in a direction of steepest entropy ascent.
What does the first law of thermodynamics state?
The laws of thermodynamics are deceptively simple to state, but they are far-reaching in their consequences. The first law asserts that if heat is recognized as a form of energy, then the total energy of a system plus its surroundings is conserved; in other words, the total energy of the universe remains constant.
See some more details on the topic Which law of thermodynamics states that energy in a system Cannot be created or destroyed but can only be converted from one form to another? here:
What is the second law of thermodynamics? | Live Science
The First Law of Thermodynamics states that energy cannot be created or destroyed; the total quantity of energy in the universe stays the same.
The laws of thermodynamics (article) | Khan Academy
Put another way, the First Law of Thermodynamics states that energy cannot be created or destroyed. It can only change form or be transferred from one …
Law of conservation of energy
The law of conservation of energy states that energy can neither be created nor destroyed – only converted from one form of energy to …
The Four Laws of Thermodynamics – Chemistry LibreTexts
The First Law of Thermodynamics states that energy can be converted from one form to another with the interaction of heat, work and internal …
What is energy conservation law?
The law of conservation of energy states that energy can neither be created nor be destroyed. Although, it may be transformed from one form to another. If you take all forms of energy into account, the total energy of an isolated system always remains constant.
What does the law of conservation state?
The law of conservation of mass states that in a chemical reaction mass is neither created nor destroyed.
What does zeroth law of thermodynamics states?
The zeroth law of thermodynamics states that if two bodies are each in thermal equilibrium with some third body, then they are also in equilibrium with each other.
What is the 3rd law of thermodynamics in simple terms?
In simple terms, the third law states that the entropy of a perfect crystal of a pure substance approaches zero as the temperature approaches zero. The alignment of a perfect crystal leaves no ambiguity as to the location and orientation of each part of the crystal.
The Laws of Thermodynamics, Entropy, and Gibbs Free Energy
Images related to the topicThe Laws of Thermodynamics, Entropy, and Gibbs Free Energy

What is the zeroth first and second law of thermodynamics?
→ The laws of thermodynamics are: First law of thermodynamics: Energy can neither be created nor be destroyed, it can only be transferred from one form to another. Second law of thermodynamics: The entropy of any isolated system always increases.
What is the 2nd law of thermodynamics in simple terms?
The second law of thermodynamics means hot things always cool unless you do something to stop them. It expresses a fundamental and simple truth about the universe: that disorder, characterised as a quantity known as entropy, always increases.
Why is it called the Zeroth Law of Thermodynamics?
The Zeroth law is called so because the first two laws of thermodynamics were established before, and they were named before. It was later found that zeroth law was more fundamental than the other laws of thermodynamics, that is the reason why it is called zeroth law of thermodynamics.
What is entropy and third law of thermodynamics?
The third law of thermodynamics states that the entropy of a perfect crystal at a temperature of zero Kelvin (absolute zero) is equal to zero. Entropy, denoted by ‘S’, is a measure of the disorder/randomness in a closed system.
What is the third law of thermodynamics quizlet?
Third Law of Thermodynamics. The third law of thermodynamics states that the entropy of a system approaches a constant value as the temperature approaches zero. Absolute zero. The coldest temperature, 0 Kelvin, that can be reached. It is the hypothetical temperature at which all molecular motion stops.
What is entropy law?
Entropy is central to the second law of thermodynamics, which states that the entropy of isolated systems left to spontaneous evolution cannot decrease with time, as they always arrive at a state of thermodynamic equilibrium, where the entropy is highest.
What is the 2nd Law of Thermodynamics and give an example?
Second law of thermodynamics:Statement,examples and applications. The second law of thermodynamics states that heat can flow spontaneously from a hot object to a cold object; heat will not flow spontaneously from a cold object to a hot object. Carnot engine, heat engine are some examples of second law of thermodynamics …
What is the fifth law of thermodynamics?
The proposed Fifth Law is an extension of the Second Law of Thermodynamics. It postulates that, as a result of wear and tear, a machine will cease functioning as the sum of all the useful energy produced approaches the total energy expended in its construction.
What is the 1st law of thermodynamics in simple terms?
The first law of thermodynamics states that energy can neither be created nor destroyed, only altered in form. For any system, energy transfer is associated with mass crossing the control boundary, external work, or heat transfer across the boundary. These produce a change of stored energy within the control volume.
When was the Zeroth Law of Thermodynamics discovered?
This might also be expressed by saying that there is precisely one kind of non-mechanical, non-matter-transferring contact equilibrium between thermodynamic systems. According to Sommerfeld, Fowler coined the term zeroth law of thermodynamics while discussing the 1935 text by Meghnad Saha and B.N. Srivastava.
Thermodynamics of materials Part 3
Images related to the topicThermodynamics of materials Part 3

What is the 1st and 2nd law of thermodynamics?
“The first law of thermodynamics also known as the law of conservation of energy states that energy can neither be created nor destroyed, but it can be changed from one form to another.” “The second law of thermodynamics states that the entropy in an isolated system always increases.
What is the difference between the first law of thermodynamics and the law of conservation of energy?
The first law of thermodynamics, also known as Law of Conservation of Energy, states that energy can neither be created nor destroyed; energy can only be transferred or changed from one form to another.
Related searches to Which law of thermodynamics states that energy in a system Cannot be created or destroyed but can only be converted from one form to another?
- what are the 3 laws of conservation of energy
- second law of thermodynamics entropy
- laws of thermodynamics pdf
- laws of thermodynamics
- the first law of thermodynamics states that
- energy cannot be created or destroyed
- what does the second law of thermodynamics state
- third law of thermodynamics
Information related to the topic Which law of thermodynamics states that energy in a system Cannot be created or destroyed but can only be converted from one form to another?
Here are the search results of the thread Which law of thermodynamics states that energy in a system Cannot be created or destroyed but can only be converted from one form to another? from Bing. You can read more if you want.
You have just come across an article on the topic Which law of thermodynamics states that energy in a system Cannot be created or destroyed but can only be converted from one form to another?. If you found this article useful, please share it. Thank you very much.