Are you looking for an answer to the topic “Which of the following has highest electron affinity of fluorine chlorine bromine iodine?“? We answer all your questions at the website Ecurrencythailand.com in category: +15 Marketing Blog Post Ideas And Topics For You. You will find the answer right below.
In electron affinity, the atom gains electrons. Fluorine, chlorine, bromine and iodine belong to the halogen family (group-17). We know that electron affinity decreases on moving down the group, but chlorine would have more affinity than fluorine because the size of fluorine is smaller than the size of chlorine.The element with the highest electron affinity among halogens is Chlorine (Cl).Fluorine has highest electron affinity in the periodic table.

Which has the highest electron affinity fluorine chlorine bromine iodine?
The element with the highest electron affinity among halogens is Chlorine (Cl).
Which of the following electrons has the highest electron affinity?
Fluorine has highest electron affinity in the periodic table.
know Electron affinity in just 4 minutes..why E.A of cl is greater than F?( for 11th,12th and bsc)..
Images related to the topicknow Electron affinity in just 4 minutes..why E.A of cl is greater than F?( for 11th,12th and bsc)..

Which has higher electron affinity fluorine or chlorine?
Electronegativity of fluorine is greater than that of chlorine but electron affinity of chlorine is greater than that of fluorine.
Which has more electron affinity chlorine or bromine?
Chlorine does have a higher electron affinity than bromine.
What is the electron affinity of bromine?
Electron Affinity of Bromine is 324.6 kJ/mol.
Chlorine has ___________ electron affinity than fluorine.
Images related to the topicChlorine has ___________ electron affinity than fluorine.

Which has greater electron affinity fluorine or bromine?
…
Electron Affinity (decreases down the group)
| Halogen | Electron Affinity (kJ/mol) |
|---|---|
| Bromine | -324.6 |
| Iodine | -295.2 |
| Astatine | -270.1 |
See some more details on the topic Which of the following has highest electron affinity of fluorine chlorine bromine iodine? here:
Which element has the highest electron affinity among …
Fluorine has the highest electron affinity among halogens. Electron affinity, also known as electronegativity, is influenced by the size of the atom,…
What is the electron affinity of iodine?
Electron Affinity of Iodine is 295.2 kJ/mol.
What is the electron affinity of chlorine?
The electron affinity of chlorine is +37 eV.
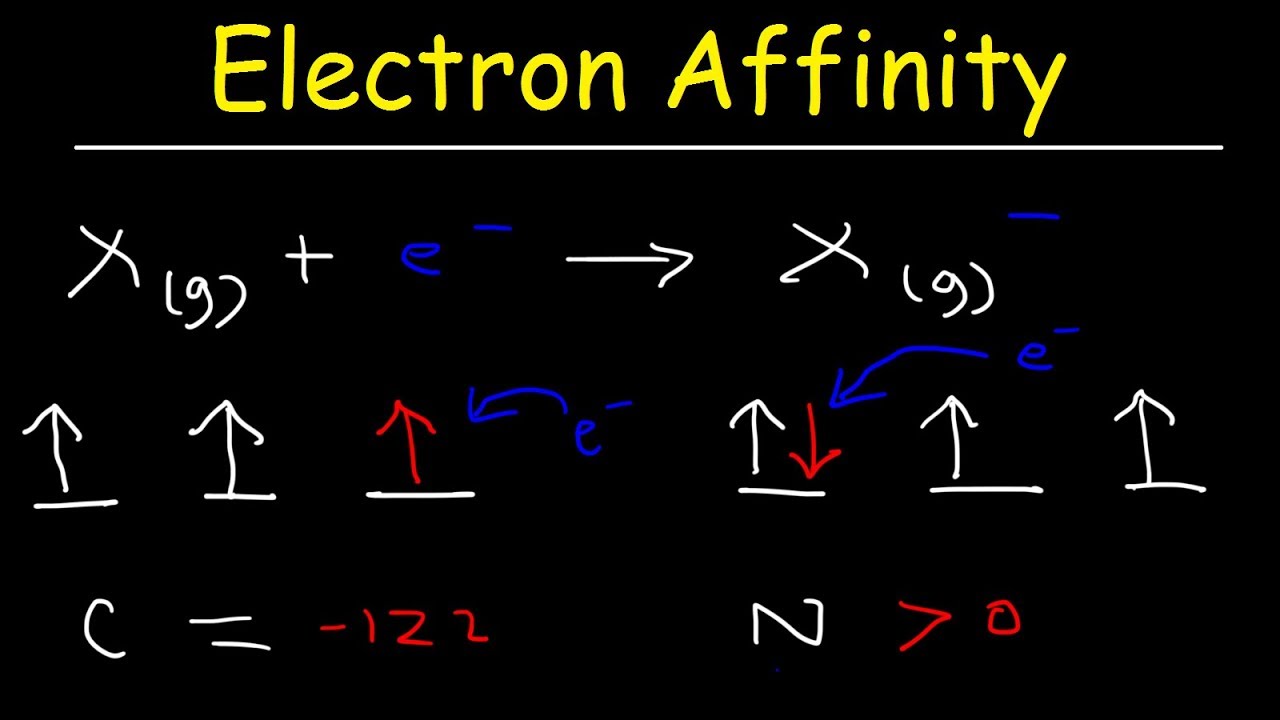
Electron Affinity Trend, Basic Introduction, Chemistry
Images related to the topicElectron Affinity Trend, Basic Introduction, Chemistry
What is the electron affinity of fluorine?
Electron Affinity of Fluorine is 328 kJ/mol.
Why iodine has a lower electron affinity than bromine?
On moving from chlorine to bromine to iodine, the electron affinity decreases (becomes less negative). This is because the increase in atomic size decrease the effective nuclear charge. Hence, the additional electron feels less attraction by the large atom. Was this answer helpful?
Related searches to Which of the following has highest electron affinity of fluorine chlorine bromine iodine?
- which group has highest electron affinity
- highest electron affinity in the halogen family is
- which has more electron affinity oxygen or sulphur
- why does cl have the highest electron affinity
- among the following which has higher electron affinity value
- noble gas has positive electron affinity
- among the following configuration the element which has the highest electron affinity is
- electron affinity of iodine
Information related to the topic Which of the following has highest electron affinity of fluorine chlorine bromine iodine?
Here are the search results of the thread Which of the following has highest electron affinity of fluorine chlorine bromine iodine? from Bing. You can read more if you want.
You have just come across an article on the topic Which of the following has highest electron affinity of fluorine chlorine bromine iodine?. If you found this article useful, please share it. Thank you very much.