Are you looking for an answer to the topic “Which Is The Formula For A Polar Molecule?“? We answer all your questions at the website Ecurrencythailand.com in category: +15 Marketing Blog Post Ideas And Topics For You. You will find the answer right below.
Water (H2O) is a polar molecule. The bonds between hydrogen and oxygen are distributed so that the hydrogen atoms are both on one side of the oxygen atom rather than evenly spaced.A polar molecule is a molecule in which one end of the molecule is slightly positive, while the other end is slightly negative. A diatomic molecule that consists of a polar covalent bond, such as HF, is a polar molecule.Water is a polar molecule due to the strong electronegativity of the oxygen atom. This forces most of the electrons to the side of the molecule where oxygen is present, creating a highly negative area.
| Formula | Description | |
|---|---|---|
| Polar | AxOH | Molecules with an OH at one end |
| OxAy | Molecules with an O at one end | |
| NxAy | Molecules with an N at one end | |
| Nonpolar | A2 | Diatomic molecules of the same element |

What formula is a polar molecule?
| Formula | Description | |
|---|---|---|
| Polar | AxOH | Molecules with an OH at one end |
| OxAy | Molecules with an O at one end | |
| NxAy | Molecules with an N at one end | |
| Nonpolar | A2 | Diatomic molecules of the same element |
What represents a polar molecule?
A polar molecule is a molecule in which one end of the molecule is slightly positive, while the other end is slightly negative. A diatomic molecule that consists of a polar covalent bond, such as HF, is a polar molecule.
Polar and NonPolar Molecules: How To Tell If a Molecule is Polar or Nonpolar
Images related to the topicPolar and NonPolar Molecules: How To Tell If a Molecule is Polar or Nonpolar

Which of the following is a polar molecule?
Water is a polar molecule due to the strong electronegativity of the oxygen atom. This forces most of the electrons to the side of the molecule where oxygen is present, creating a highly negative area.
Is CH4 polar?
So, is CH4 polar or nonpolar? CH4 is a nonpolar molecule as it has a symmetric tetrahedral geometrical shape with four identical C-H bonds. The electronegativity of carbon and hydrogen is 2.55 and 2.2, respectively, which causes the partial charges to be almost zero.
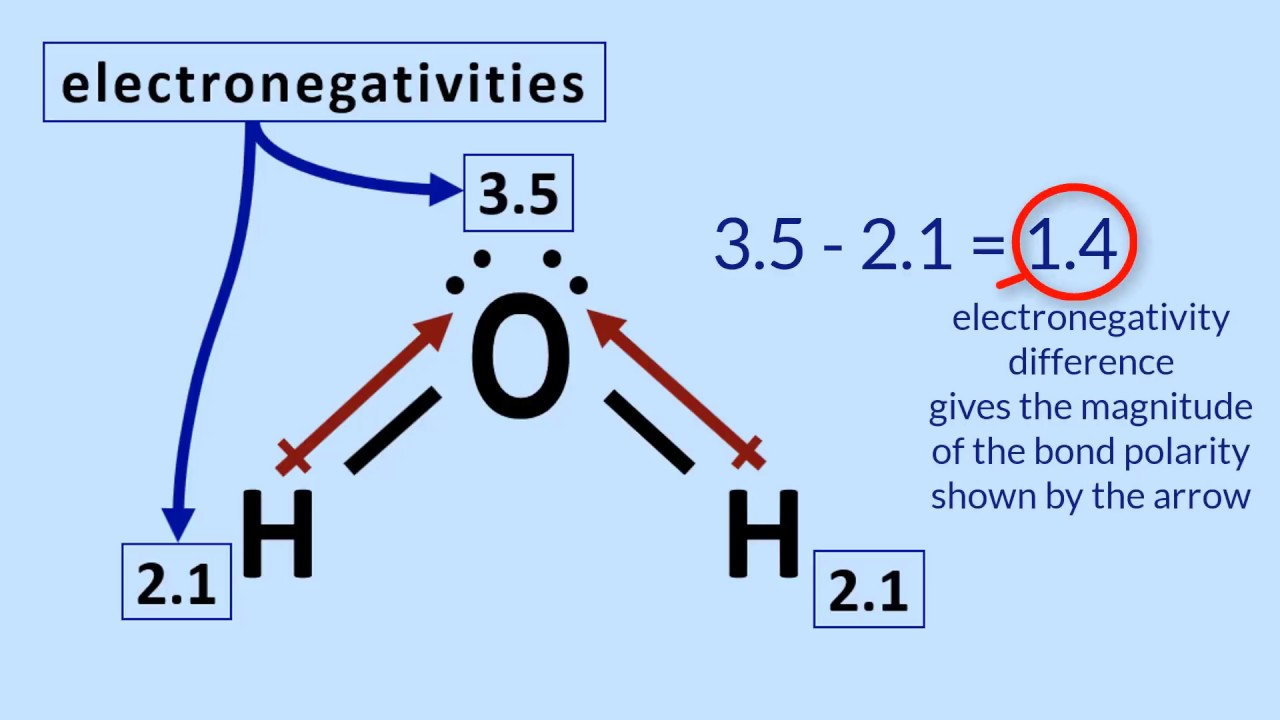
Is H2O a polar molecule?
Water (H2O), like hydrogen fluoride (HF), is a polar covalent molecule.
Is NH3 a polar molecule?
Yes, NH3 (Ammonia) molecule is polar in nature because of its asymmetrical shape ie; trigonal pyramidal structure, and the difference in electronegativities of N(3.04) and H(2.2).
Is CCl4 polar?
CCl4 that is carbon tetrachloride is nonpolar because all the four bonds are symmetrical, and they are they extended in all the directions.
See some more details on the topic Which Is The Formula For A Polar Molecule? here:
4.12: Shapes and Properties- Polar and Nonpolar Molecules
A polar molecule is a molecule in which one end of the molecule is slightly positive, while the other end is slightly negative. A diatomic …
Molecular Polarity
The 0.49 difference in electronegativity for the C-N bond tells us that it is polar. Molecules with one polar bond are always polar.
covalent naming and lewis structures Flashcards | Quizlet
which formula represents a nonpolar molecule containing polar covalent bonds? a. H2O b. CCl4 c. NH3 d. H2. b. CCl4.
Is NaCl polar or nonpolar?
Sodium Chloride (NaCl) which is an ionic compound acts as a polar molecule. Usually, the large difference in electronegativities in sodium and chlorine makes their bond polar.
What are polar molecules give one example?
Polar molecules: The molecules in which “centre of gravity” of positive nuclei and revolving electrons do not coincide are known as polar molecules. Example: HCl, H2O, N2O etc. Polar substances behave as a tiny electric dipole because polar molecules have a permanent electric dipole moment.
Is o2 polar or nonpolar?
Diatomic oxygen is made up of the same two elements, and they equally share the 4 electrons that make up the double bond between them. They’re equally electromagnetic, which means that there are not any partial charges for each element. Since neither atom pulls harder, it’s a non- polar covalent bond.
Is co2 polar?
Carbon dioxide is a linear molecule while sulfur dioxide is a bent molecule. Both molecules contain polar bonds (see bond dipoles on the Lewis structures below), but carbon dioxide is a nonpolar molecule while sulfur dioxide is a polar molecule.
Is Cl2 polar or nonpolar?
Cl2 (Chlorine) is nonpolar in nature because of its linear symmetrical shape and it consists of two chlorine atoms having equal electronegativity. As a result, both atoms have equal charge distribution on them, and the molecule results in zero dipole moment that makes the chlorine molecule nonpolar.
Polar Molecules Tutorial: How to determine polarity in a molecule
Images related to the topicPolar Molecules Tutorial: How to determine polarity in a molecule
Is Br2 a polar molecule?
So, Is Br2 Polar or Nonpolar? Br2 (Bromine) is nonpolar because, in this molecule, both bromine atoms have the same electronegativity due to which both atoms have equal charge distribution and results in a net-zero dipole moment. It is linear in structure.
Is SF4 polar?
The Sulfur tetrafluoride is a polar molecule because Fluorine is more electronegative than Sulfur. With this, the distribution of the charge is not equal, making the SF4 polar molecules. The molecular geometry of SF4 is in a seesaw molecular shape that can be seen when you draw the Lewis Structure.
Is BF3 polar?
Boron trifluoride (BF3) is a nonpolar molecule, whereas ammonia (NH3) is a polar molecule.
Is SO3 polar?
Because the valence electrons in sulfur trioxide (SO3) are shared equally in the molecular structure, it is a nonpolar molecule, and the Lewis structure of SO3 appears to be a well symmetrical structure. Its trigonal planar form, sulfur trioxide (SO3) is a nonpolar molecule.
Is HCl polar?
Is HCl polar or nonpolar? Because chlorine is more electronegative than hydrogen, hydrochloric acid HCl forms a polar bond, and is therefore a polar molecule. There is no symmetry which could cancel out the dipole charge.
Is SO2 polar?
SO2 is polar in nature because of the difference in electronegativity between sulfur and oxygen atoms. The greater the difference in electronegativity more will be the polarity of the molecule. The bent shape of SO2 is because of the repulsion between the unbonded electrons present on the sulfur and oxygen atoms.
Is NF3 polar?
The molecule BF3 and NF3 , both are covalent compounds but BF3 is non – polar and NF3 is polar.
Is CHCl3 polar?
Yes, CHCl3 is polar due to its tetrahedral molecular structure and difference between the electronegativity of C, H and, CL.
Is HCN polar?
HCN, or hydrogen cyanide, is a polar molecule because there is a large electronegative difference between the N and H across the linear molecule.
Is CS2 polar?
CS2 (Carbon disulfide) is nonpolar because of its symmetric (linear) shape. Although carbon and sulfur differ in their electronegativity and C-S bond is polar, the polarity of both opposite C-S bonds gets canceled by each other resulting in a nonpolar molecule.
Polar Non-Polar Molecules: Crash Course Chemistry #23
Images related to the topicPolar Non-Polar Molecules: Crash Course Chemistry #23

Is C2H6 polar or nonpolar?
Ethane or C2H6 is a nonpolar molecule because: There is significantly less difference in the electronegativities of Hydrogen and Carbon forming bonds in this structure. There is 0 difference in electronegativity of the Carbon atoms forming bonds with each other.
Is XeF4 polar or nonpolar?
XeF4 is nonpolar. Using the Lewis Structure, we can identify the molecular geometry of XeF4. The lone pairs of electrons and the bond angles of all the atoms created nonpolar molecules in noble gases like Xe. The F-Xe-F bond angle is 90 degrees, and it creates a square planar shape.
Related searches to Which Is The Formula For A Polar Molecule?
- what is a polar molecule
- polar and nonpolar molecules examples
- formula represents a polar molecule
- what is non polar molecule
- polar vs nonpolar
- example of nonpolar molecule
- example of polar molecule
- which is the formula for a polar molecule in the diagram below
- how to tell if a molecule is polar or nonpolar without electronegativity
Information related to the topic Which Is The Formula For A Polar Molecule?
Here are the search results of the thread Which Is The Formula For A Polar Molecule? from Bing. You can read more if you want.
You have just come across an article on the topic Which Is The Formula For A Polar Molecule?. If you found this article useful, please share it. Thank you very much.