Are you looking for an answer to the topic “What is the atomic mass of neon give your answer to the nearest 10th?“? We answer all your questions at the website Ecurrencythailand.com in category: +15 Marketing Blog Post Ideas And Topics For You. You will find the answer right below.
The periodic table shows atomic mass of Ne to be 20.180. Rounding this to a whole number you get 20 g/mole.Neon has an atomic number of 10 and a mass number of 20.1797. Neon, Ne, is a noble gas and it is a gas that fills neon signs. It is element #10 in the periodical table. Its relative atomic mass is 20.1797.Atomic mass of Neon is 20.1797 u.
Note that each element may contain more isotopes.

What is the atomic mass of neon nearest 10?
Neon has an atomic number of 10 and a mass number of 20.1797. Neon, Ne, is a noble gas and it is a gas that fills neon signs. It is element #10 in the periodical table. Its relative atomic mass is 20.1797.
What is the atomic mass of neon 12?
Atomic mass of Neon is 20.1797 u.
Note that each element may contain more isotopes.
Calculating the Average Atomic Mass for Neon- Example
Images related to the topicCalculating the Average Atomic Mass for Neon- Example

How do you find the atomic mass of neon?
Actually Neon would be lested as having an atomic mass of about 20.18 because of a mixute of isotopes. Z= Atomic number the number of protons in the nucleus which defines the element. in this case Z=10 and this means neon but there is also 10 neurons in the nucleus of the major isotope whcich gives it ^20Ne.
What is the atomic number of neon Ne?
What is the mass of 1 atom of neon?
1, and the atomic mass of neon (symbol Ne) is 20.8, which is what we calculated in Example 4.9.
How do u calculate atomic mass?
Together, the number of protons and the number of neutrons determine an element’s mass number: mass number = protons + neutrons. If you want to calculate how many neutrons an atom has, you can simply subtract the number of protons, or atomic number, from the mass number.
Why is the atomic mass of neon?
Hence, the atomic mass of Neon is 20.18 amu.
See some more details on the topic What is the atomic mass of neon give your answer to the nearest 10th? here:
Periodic Table – Assignment Flashcards | Quizlet
What is the atomic mass of neon? Give your answer to the nearest tenth. 10, 20.2.
Mass numbers – The Periodic Table
Neon has the Atomic Mass weight of 20.18. Round to the nearest whole number. The mass number of neon is therefore 20. Mass Numbers = Atomic Weight of Element, …
2.3: Calculating Atomic Masses – Chemistry LibreTexts
Define the atomic mass unit and average atomic mass; Calculate average … The average mass of a neon atom in the solar wind is 20.15 amu.
Neon – Element information, properties and uses – The Royal …
Element Neon (Ne), Group 18, Atomic Number 10, p-block, Mass 20.180. Sources, facts, uses … This gives a fraction that contains both helium and neon.
How many protons does neon 12 have?
The preceding numbers are for neon, but all elements have their own unique percent natural abundance of isotopes. For neon, which has 10 protons, the mass numbers of the three different naturally occurring isotopes are 20, 21, and 22, corresponding to 10, 11, and 12 neutrons, respectively.
How many electrons does neon 12 have?
Follow-Up #1: Atomic properties of Neon
There are 10 protons, and 10 electrons in un-ionized neon. 90% of the neon atoms contain 10 neutrons. There are a few with 11 neutrons and around 9% with 12.
What mass of neon should be?
The average atomic mass of Neon is 20.18 amu.
Calculate the Mass of a Single Atom or Molecule
Images related to the topicCalculate the Mass of a Single Atom or Molecule
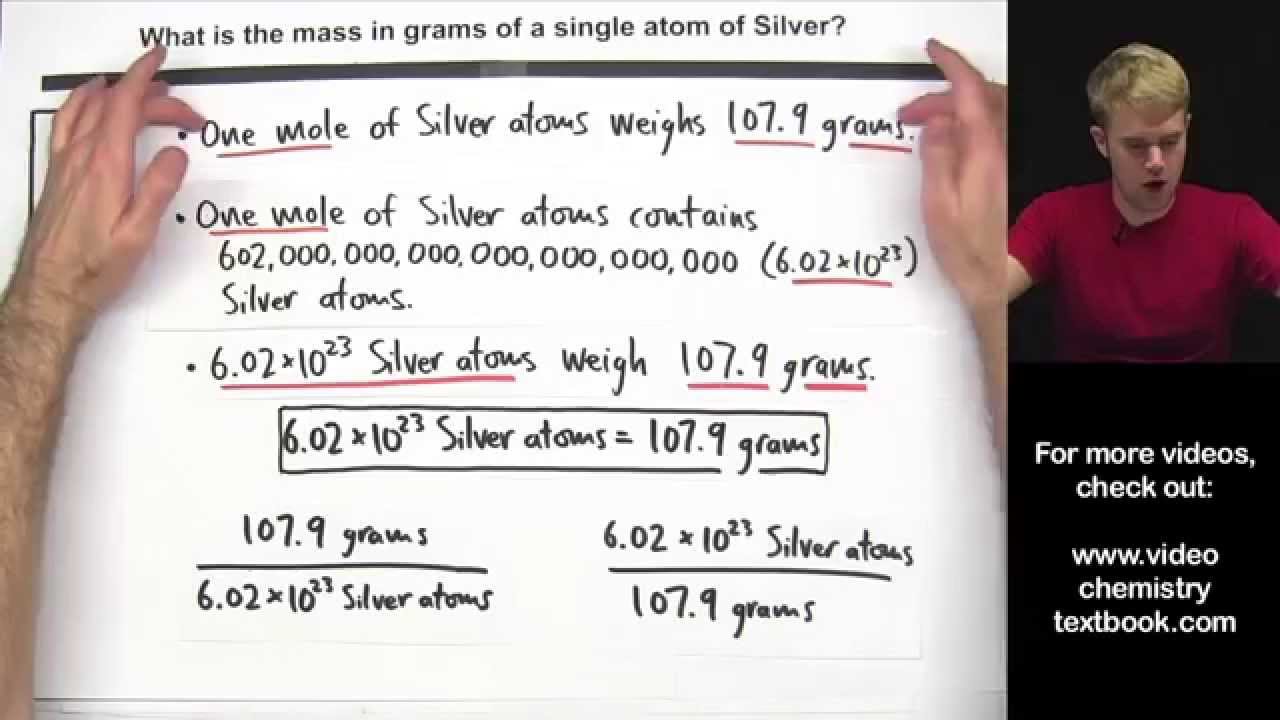
Why is the atomic mass of neon in a fraction?
Reason for fractional atomic mass:
The atomic masses of most elements are fractional because they exist as a mixture of isotopes of different masses. Since the atomic mass of isotopes are different, so average atomic mass is calculated for a single element.
What is the mass number of 21?
| ATOMIC NUMBER | ELEMENT | ATOMIC MASS |
|---|---|---|
| 21 | Scandium | 44.956 |
| 22 | Titanium | 47.867 |
| 23 | Vanadium | 50.942 |
| 24 | Chromium | 51.996 |
What is the 10th element?
The tenth element on the periodic table is Neon (Ne).
What is the number of protons of neon atom which has an atomic number 10?
| Neon(Ne) atomic number | 10 |
|---|---|
| Protons | 10 |
| Neutrons | 10 |
| Group | 18 |
| Period | 2 |
What is the configuration of neon 10?
Neon is the tenth element with a total of 10 electrons. In writing the electron configuration for neon the first two electrons will go in the 1s orbital. Since 1s can only hold two electrons the next 2 electrons for Ne go in the 2s orbital. The remaining six electrons will go in the 2p orbital.
How many neutrons are in neon?
Neon always has 10 protons, but the number of neutrons varies with each isotope. The most common are 2010Ne (10 protons, 90.48% abundance), 2110Ne (11 protons, . 27% abundance), and 2210Ne (12 protons, 9.25% abundance).
What is the symbol for neon?
neon (Ne), chemical element, inert gas of Group 18 (noble gases) of the periodic table, used in electric signs and fluorescent lamps.
What is the charge of neon?
| Number | Element | Charge |
|---|---|---|
| 7 | nitrogen | 3- |
| 8 | oxygen | 2- |
| 9 | fluorine | 1- |
| 10 | neon | 0 |
What is atomic mass Class 9 CBSE?
Atomic mass refers to the mass of an atom. It depicts how many times an atom of an element is heavier than one-twelth (1/12th) the mass of one atom of carbon-12 of mass of one carbon atom. The relative atomic masses of all elements have been established with reverence to an atom of carbon-12.
CIE AS level Chemistry 9701 | S19 Q11 | Fully Solved Paper | May/June 2019 Qp 11 | 9701/11/M/J/19
Images related to the topicCIE AS level Chemistry 9701 | S19 Q11 | Fully Solved Paper | May/June 2019 Qp 11 | 9701/11/M/J/19

What is called atomic mass?
atomic mass, the quantity of matter contained in an atom of an element. It is expressed as a multiple of one-twelfth the mass of the carbon-12 atom, 1.992646547 × 10−23 gram, which is assigned an atomic mass of 12 units. In this scale, 1 atomic mass unit (amu) corresponds to 1.660539040 × 10−24 gram.
What is atomic mass give example?
It is approximately equivalent to the number of protons and neutrons in the atom (the mass number) or to the average number allowing for the relative abundances of different isotopes. Foe example: Atomic mass of Na is 23 u. Sodium has 11 protons and 12 neutrons, hence it is equal to 11 + 12 = 23.
Related searches to What is the atomic mass of neon give your answer to the nearest 10th?
- how many neutrons does an atom of zinc contain give your answer to the nearest whole number
- why might calcium be important in the diet of many living things?
- what is the identity of an atom with 76 protons 41 neutrons and 70 electrons
- what is the period number in which helium is found
- what is the identity of an atom with 76 protons, 41 neutrons, and 70 electrons?
- how many neutrons does neon have
- what subatomic atomic particle determines the identity of the atom
- how many neutrons does an atom of zinc contain? give your answer to the nearest whole number.
- how many protons does zinc have
- what is the atomic mass of neon give your answer to the nearest 10th
- what is the atomic mass of silver
- how many neutrons are in an atom that has an atomic number of 45 and a mass number of 62?
- why might calcium be important in the diet of many living things
- what is the group name in which helium is found?
Information related to the topic What is the atomic mass of neon give your answer to the nearest 10th?
Here are the search results of the thread What is the atomic mass of neon give your answer to the nearest 10th? from Bing. You can read more if you want.
You have just come across an article on the topic What is the atomic mass of neon give your answer to the nearest 10th?. If you found this article useful, please share it. Thank you very much.