Are you looking for an answer to the topic “What is the n value for the 4d orbital?“? We answer all your questions at the website Ecurrencythailand.com in category: +15 Marketing Blog Post Ideas And Topics For You. You will find the answer right below.
For a 4d orbital, the value of n (principal quantum number) will always be 4 and the value of l (
) will always be equal to 2.Hence, the magnetic quantum number in 4s orbital is 0.The number before the orbital name (such as 2s, 3p, and so forth) stands for the principal quantum number, n. The letter in the orbital name defines the subshell with a specific angular momentum quantum number l = 0 for s orbitals, 1 for p orbitals, 2 for d orbitals.

What is the n value of 4s orbital?
Hence, the magnetic quantum number in 4s orbital is 0.
What is n for d orbital?
The number before the orbital name (such as 2s, 3p, and so forth) stands for the principal quantum number, n. The letter in the orbital name defines the subshell with a specific angular momentum quantum number l = 0 for s orbitals, 1 for p orbitals, 2 for d orbitals.
Example- Writing all sets of 4 Quantum Numbers for the 4d subshell
Images related to the topicExample- Writing all sets of 4 Quantum Numbers for the 4d subshell

How many electrons are in a 4d orbital?
The fourth energy level of the periodic table includes the 4s 3d and 4p orbitals. The 4p orbital holds 6 electrons. There is a 4d orbital with 10 electrons which coincides with the 5th energy level of the periodic table.
What is the magnetic quantum number of n 4?
For example, if n = 4 and l = 3 in an atom, the possible values of the magnetic quantum number are -3, -2, -1, 0, +1, +2, and +3. The total number of orbitals in a given subshell is a function of the ‘l’ value of that orbital.
What are the n and L values for 4s electrons?
for 4s, n and l are 4 and 0 respectively.
What are the n and L values of 4f orbitals?
The possible values of n, l andml quantum numbers for 4 f orbital are : n=4,L=3,ml=-3,-2,-1,0,+1,+2,+3.
What is the correct representation for an orbital which has an n value of 4 and an L value of 2?
Answer and Explanation: The quantum numbers n = 4 and l = 2 correspond to 4d subshell.
See some more details on the topic What is the n value for the 4d orbital? here:
How many electrons are present in a 4d orbital?
What are the four quantum numbers for an electron in a 4d orbital? … For a 4d orbital, the value of n (principal quantum number) will always be …
Quantum Numbers, Atomic Orbitals, and Electron Configurations
The total number of orbitals for a given n value is n2. … 1s, 2s, 2p, 3s, 3p, 4s, 3d, 4p, 5s, 4d, 5p, 6s, 4f, 5d, 6p, 7s, 5f.
Quantum Numbers and Electronic Structure
For example, when l = 0, the orbital is spherical. For each principal energy level (designated by n) there are n sublevels (i.e., n values of l): l=0,1,2,3, …
What is value for d orbitals?
Once principle quantum number n equals 3 or greater, angular quantum number can equal 2. When angular quantum number l=2, it is considered the d-orbital. For the d-orbital, the magnetic quantum number ml can equal -2 to 2, taking the possible values -2, -1, 0, 1, or 2.
What is n 1 in d block?
n is considered to be the principal quantum number of the last shell. (n-1) is principal quantum number of the shell before the last shell i.e. penultimate shell. So, (n-1) d orbital means the d orbital in penultimate shell. Hope, it helps. 4.4K views.
When 4d orbital is complete?
Solution. After filling the 4d-orbital, an electron will enter in 5p orbital. As the value of (n+l) for 4d orbital is same as that of 5p orbital, 4d orbital is filled before 5p orbital as 4d orbital has lower value of n than 5 p orbital.
The shape of the 4d orbital — Chemistry X
Images related to the topicThe shape of the 4d orbital — Chemistry X

What are the quantum numbers for 4d?
For a 4d orbital, the value of n (principal quantum number) will always be 4 and the value of l (azimuthal quantum number) will always be equal to 2. The values of the magnetic quantum number range from -l to l, so the possible values of ml for the 4d orbital are -2, -1, 0, 1, and 2.
How many Subshells are in the n 4 shell?
Solution. The four sub-shells are associated with n = 4, which are s, p, d and f. The number of orbitals = 16.
How many orbitals are there in n 3?
For n = 3 there are nine orbitals, for n = 4 there are 16 orbitals, for n = 5 there are 5 2 = 25 orbitals, and so on. How many electrons does n 4 l 3 have? As you know, the number of orbitals you get per energy shell is given by the equation no.
What is the value of 4s?
Answer. The value of n in 4s orbital is 4 and the value of l in 4s orbital is 0.
What are the values of n and l for 5f orbital?
Choice “a” is n = 5, l = 3, ml = +1, which is the 5f orbital…
What is the n 1 value of 3p orbital?
CORRECT: For the 3p sublevel, the principal quantum number (n) is 3 and the angular momentum quantum number (l) is 1.
What is the value of n 1 for 4f electron?
For a 4f orbital, the principal quantum number is n = 4, the azimuthal quantum number is 3. The values of magnetic quantum numbers will be -3, -2, -1, 0, +1, +2, +3. Total 7 orbitals are present in 4f subshell.
How many orbitals are in 4f?
For any atom, there are seven 4f orbitals. The f-orbitals are unusual in that there are two sets of orbitals in common use. The first set is known as the general set, this page.
What are the n and L quantum numbers for the 4p subshell?
| n | l | Orbital Name |
|---|---|---|
| 4 | 0 | 4s |
| 1 | 4p | |
| 2 | 4d | |
| 3 | 4f |
4d orbital quantum numbers
Images related to the topic4d orbital quantum numbers
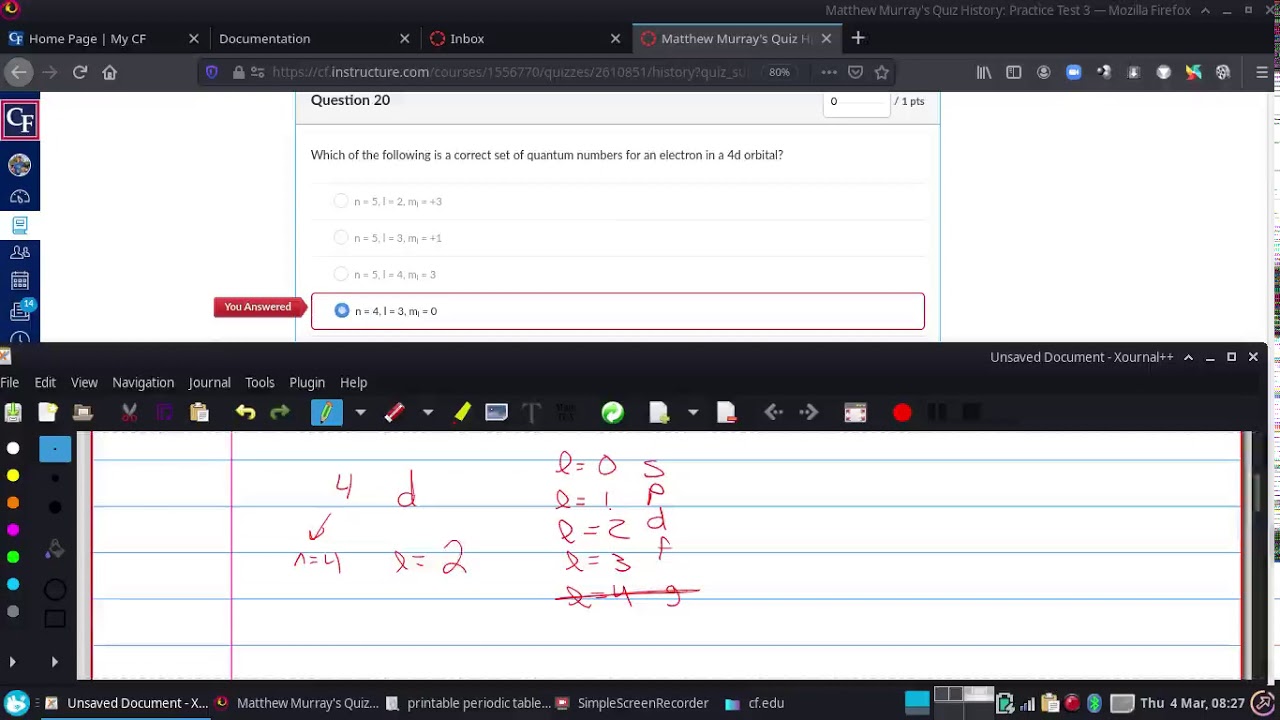
When n is equal to 4 and L is equal to 3 the orbital is?
Since here it’s provided as l=3 so it’s a cloverleaf. The designation of orbital is 4f.
What is the maximum number of electrons that can be represented by n 4?
Therefore in n=4, number of subshells=4, orbitals=16 and number of electrons =32.
Related searches to What is the n value for the 4d orbital?
- 4d orbital shape
- 5f n and l values
- what is the n value for the 4d orbital
- 4d orbital electrons
- what is the value of n the principal quantum number for a 4d orbital
- what is the value of the spin quantum number for an electron in a 4d orbital
- what are the values of n and l for 4d orbital
- 4d orbital n and l
- there are five 4d orbitals list the quantum numbers for each orbital
- list the values of n l and m for orbitals in the 4d subshell
- list the values of n, l, and m/ for orbitals in the 4d subshell.
- 4d orbital nodes
Information related to the topic What is the n value for the 4d orbital?
Here are the search results of the thread What is the n value for the 4d orbital? from Bing. You can read more if you want.
You have just come across an article on the topic What is the n value for the 4d orbital?. If you found this article useful, please share it. Thank you very much.