Are you looking for an answer to the topic “What is the effect of temperature on Henry’s law constant KH on solubility of a gas in liquid?“? We answer all your questions at the website Ecurrencythailand.com in category: +15 Marketing Blog Post Ideas And Topics For You. You will find the answer right below.
Solubility of a gas in liquid decreases with increase in temperature. KH value increases with the increase in temperature.The volume of given mass of dissolved gas in solution also increases with increase of temperature. It becomes impossible for solvent to accommodate gaseous solute in it and gas bubbles out. Hence, with increase in temperature, the solubility of a gas in a liquid decreases.The Henry’s law constant decreased linearly as the temperature increased and the pressure decreased. The Henry’s law constant was found to be more strongly dependent on the temperature than on the pressure.
What is the effect of temperature on Henry’s law constant for the solubility of a gas in a solvent keeping the pressure of gas same?
The volume of given mass of dissolved gas in solution also increases with increase of temperature. It becomes impossible for solvent to accommodate gaseous solute in it and gas bubbles out. Hence, with increase in temperature, the solubility of a gas in a liquid decreases.
What is the effect of temperature on Henry’s law constant?
The Henry’s law constant decreased linearly as the temperature increased and the pressure decreased. The Henry’s law constant was found to be more strongly dependent on the temperature than on the pressure.
Henry’s Law and Gas Solubility Explained
Images related to the topicHenry’s Law and Gas Solubility Explained

What is the effect of temperature on the solubility of a gas in liquid?
The solubility of gases in liquids decreases with increasing temperature. Conversely, adding heat to the solution provides thermal energy that overcomes the attractive forces between the gas and the solvent molecules, thereby decreasing the solubility of the gas; pushes the reaction in Equation 4 to the left.
What is the relationship between KH and solubility?
Henry was the first to give a quantitative relation between pressure and solubility of a gas in a solvent which is known as Henry’s law. The law states that at a constant temperature, the solubility (S) of a gas in a liquid is directly proportional to the pressure (P) of the gas.
What is the effect of temperature on solubility?
In general, solids become more soluble as the temperature increases. This is why sugar dissolves better in hot water than in cold water. The table shows three examples of the solubility (g of solute per 100 g water) of substances at different temperatures.
How does Henry’s constant KH of a gas in a particular solvent vary with temperature?
KH depends only on nature of gas, nature of liquid and temperature (T). As temperature increases, ‘KH ‘ increases and X decreases.
Why does Henry’s constant increases with increase in temperature?
(a) Value of Henry’s constant (KH) increases with increase in temperature representing the decrease in solubility.
See some more details on the topic What is the effect of temperature on Henry’s law constant KH on solubility of a gas in liquid? here:
What is the effect of temperature on Henry’s law constant (KH …
Solubility of a gas in liquid decreases with increase in temperature. KH value increases with the increase in temperature.
State Henry’s law. What is the effect of temperature on … – Toppr
Henry’s law states that the solubility of a gas in a liquid at constant temperature is proportional to the pressure of the gas above the solution.
Henry’s law of solubility of gas in liquid – The Fact Factor
The solubility of gases in liquids decreases with the rise in temperature. When dissolved, the gas molecules are present in the liquid phase and …
Henry’s law – Knowino
At a constant temperature, the amount of a given gas dissolved in a given type and volume of …
What is the effect of change in temperature on the solubility of a solid in liquid B gas in liquid?
The solubility of most substances depends strongly on the temperature and, in the case of gases, on the pressure. The solubility of most solid or liquid solutes increases with increasing temperature.
What is the effect of temperature on solubility of solid in liquid?
For many solids dissolved in liquid water, the solubility increases with temperature. The increase in kinetic energy that comes with higher temperatures allows the solvent molecules to more effectively break apart the solute molecules that are held together by intermolecular attractions.
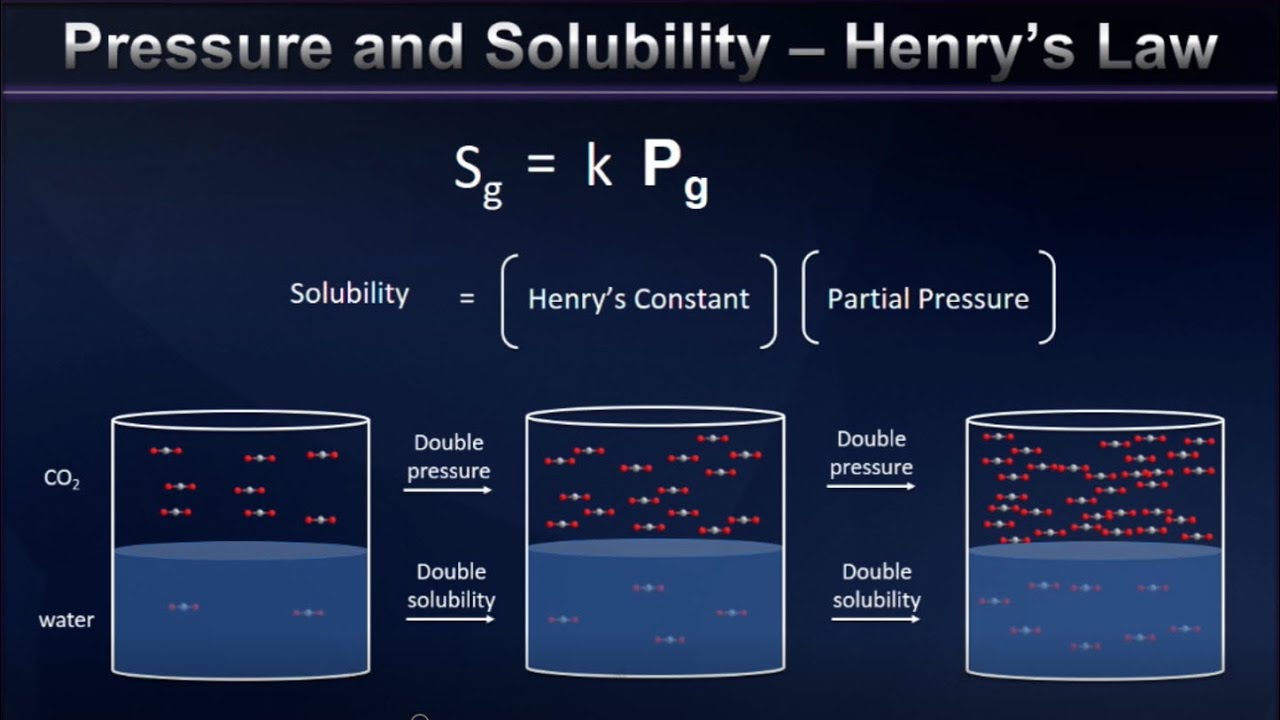
Pressure and Gas Solubility (Henry’s Law)
Images related to the topicPressure and Gas Solubility (Henry’s Law)

What is Henry’s law write significance of KH Why do gases always tend to be less soluble in liquid as the temperature is raised?
Henry’s law states that the partial pressure of a gas in vapour phase is proportional to the mole fraction in the solution. Gases tend to be less soluble in liquids as the temperature is raised because the dissolution of gas in a liquid is an exothermic process and the heat is evolved during this process.
What is the effect of temperature on the solubility of gases in liquids Class 11?
Solubility of gases in liquids decreases with increase in temperature.
What is the effect of temperature on the solubility of gas in liquid Class 12?
Hence, solubility of gas in liquid decreases with increase in temperature.
Does solubility increase with Henry’s constant?
Pressure has very little effect on the solubility of solids or liquids, but has a significant effect on the solubility of gases. Gas solubility increases as the partial pressure of a gas above the liquid increases.
Why Henry constant is inversely proportional to solubility?
According to henry’s law, the mole fraction of gas soluble in water is directly proportional to the pressure applied on the solution . As value of Kh increases mole fraction of gas soluble in solvent decreases. Kh increases with increases in temperature so solubility decreases with increase in temperature.
What is KH in Henry’s Law?
This air-water distribution ratio (Kaw) is also referred to as the Henry’s Law constant KH. The Henry’s Law constant KH can be approximated as the ratio of a compound’s abundance in the gas phase to that in the aqueous phase at equilibrium.
How the temperature can affect in solubility of solid and gases?
Sparingly soluble solid or liquid substances can be dissolved completely by increasing the temperature. But in case of gaseous substance, temperature inversely influences solubility i.e. as the temperature increases gases expand and escapes from their solvent.
Henry’s Law Explained – Gas Solubility Partial Pressure – Chemistry Problems
Images related to the topicHenry’s Law Explained – Gas Solubility Partial Pressure – Chemistry Problems

What affects the solubility of a gas?
Solubility is the maximum amount of a substance that will dissolve in a given amount of solvent at a specific temperature. There are two direct factors that affect solubility: temperature and pressure. Temperature affects the solubility of both solids and gases, but pressure only affects the solubility of gases.
Which of the following is correct about Henry’s law constant KH?
Solution: The correct answer is option B. At a constant pressure KH is inversely proportional to x therefore higher the KH lower the solubility. A solution in which no more solute can be dissolved at a given temperature and pressure is called a saturated solution.
Related searches to What is the effect of temperature on Henry’s law constant KH on solubility of a gas in liquid?
- does henrys law constant change with temperature
- how does an increase in pressure affect the solubility of a solid or liquid
- henry’s law constant for oxygen in water at different temperatures
- does henry’s law constant change with temperature
- henrys law constant for oxygen in water at different temperatures
- how does an increase in pressure affect the solubility of a solid or liquid?
- what is the effect of temperature on solubility of solid in liquid
Information related to the topic What is the effect of temperature on Henry’s law constant KH on solubility of a gas in liquid?
Here are the search results of the thread What is the effect of temperature on Henry’s law constant KH on solubility of a gas in liquid? from Bing. You can read more if you want.
You have just come across an article on the topic What is the effect of temperature on Henry’s law constant KH on solubility of a gas in liquid?. If you found this article useful, please share it. Thank you very much.