Are you looking for an answer to the topic “Which element is used in dry cells?“? We answer all your questions at the website Ecurrencythailand.com in category: +15 Marketing Blog Post Ideas And Topics For You. You will find the answer right below.
A standard dry cell comprises a zinc anode, usually in the form of a cylindrical pot, with a carbon cathode in the form of a central rod. The electrolyte is ammonium chloride in the form of a paste next to the zinc anode.The correct answer is Ammonium chloride and zinc chloride. CONCEPT: The dry cell is a type of battery used in electric devices. As it uses electrolyte in the form of a paste, it can be used in portable devices.Zinc is a medium reactive metal and is oxidized slowly by air.It is electropositive as compared to carbon which is used as positive electrode in dry cell.So, it donates electrons and a current is produced. It is not very reactive and is consumed slowly, so it is used in dry cells.

Is nacl used in dry cell?
The correct answer is Ammonium chloride and zinc chloride. CONCEPT: The dry cell is a type of battery used in electric devices. As it uses electrolyte in the form of a paste, it can be used in portable devices.
Why Zn is used in dry cell?
Zinc is a medium reactive metal and is oxidized slowly by air.It is electropositive as compared to carbon which is used as positive electrode in dry cell.So, it donates electrons and a current is produced. It is not very reactive and is consumed slowly, so it is used in dry cells.
Dry Cell – Definition, Working Principle, History, Parts of Dry Cell, Chemical reactions
Images related to the topicDry Cell – Definition, Working Principle, History, Parts of Dry Cell, Chemical reactions

Which electrolyte is used in dry cell?
A standard dry cell comprises a zinc anode, usually in the form of a cylindrical pot, with a carbon cathode in the form of a central rod. The electrolyte is ammonium chloride in the form of a paste next to the zinc anode.
What is inside a dry cell?
A dry-cell battery is a device made of one or more electrochemical cells that convert stored chemical energy into electrical energy. It contains an electrolyte that is contained within a paste or other moist medium. A Standard dry cell battery includes a zinc anode and a carbon cathode within a central rod.
Why ammonium chloride is used in dry cell?
Function of ammonium chloride in dry cell batteries is to regulate the pH of electrodes by decomposing into ammonia gas and hydrogen ions. It also increases the concentration of hydrogen ions, which allows the voltage of batteries to drop.
What is the role of MnO2 in a dry cell?
MnO2 is used in the dry cell because it acts as a depolariser. It is also used because it produces moisture thereby keeping the solution moist so that the ionic mobility increases.
What is carbon zinc used for?
Uses. Zinc–carbon batteries are a reliable source of power for appliances that consume little energy, like remote controls for television, clocks, smoke detectors and flashlights. Zinc-carbon batteries were in common use with hand-cranked telephone magneto phones, powering the microphone and speaker.
See some more details on the topic Which element is used in dry cells? here:
Dry Cells – an overview | ScienceDirect Topics
Zinc–carbon batteries or ‘dry’ cells are galvanic cells that have been well known for 140 years. There are two types of zinc–carbon batteries in use today, the …
Structure of a Dry Cell – Sciencing
A dry cell is an electrochemical cell that uses a low-moisture electrolyte instead of a liquid electrolyte as a wet cell does.
Dry Battery – NEMA
A Standard dry cell battery includes a zinc anode and a carbon cathode within a central rod. Cadmium, carbon, lead, nickel, and zinc are used to manufacture …
What is a Dry Cell : Structure & Its Working – ElProCus
A dry cell is the simplest form of electricity-producing source. A number of cells combined cells together forms a battery. The lead-acid or nickel-cadmium …
Is Copper is used in dry cell?
Most commonly the dry cell battery is the zinc carbon battery. And the cell is made up of an outer container, one anode, one cathode and electrolyte are filled in the container. Thus, the electrolyte used in the dry cell is ammonium chloride. Hence, the option (D) is the correct answer.
Working of Dry Cell- Everyday Science
Images related to the topicWorking of Dry Cell- Everyday Science

Which electrolyte is used in a dry cell class 8?
Hence Electrolyte used in dry cell is Ammonium chloride i.e. NH4Cl.
What is the electrolyte used in a cell?
Commonly used electrolytes in electrolytic cells include water (containing dissolved ions) and molten sodium chloride.
How is dry cells made?
A dry cell consists of a metal container in which a low moisture electrolyte paste covers the graphite rod or a metal electrode. Generally, the metal container will be zinc whose base acts as a negative electrode (anode) and a carbon road acts as a positive electrode (cathode).
How do you make dry cells?
It’s easy to make a simple dry-cell battery to demonstrate the nature of generating electricity. You don’t need any special equipment or potentially harmful acid liquids, just spare change and salt water. Soak the coffee filter in a solution of 1 tablespoon salt to 1 cup warm water. Gently wring out most of the water.
What chemicals are in batteries?
Sodium chloride, chloric acid, nitric acid, potassium nitrate, hydrochloric acid, potassium nitrate, sulfuric acid, sodium hydroxide, magnesium hydroxide, and sodium acetate are all electrolyte compounds.
Which ammonium salt is used in dry cell?
The salt used in dry cell is ammonium chloride.
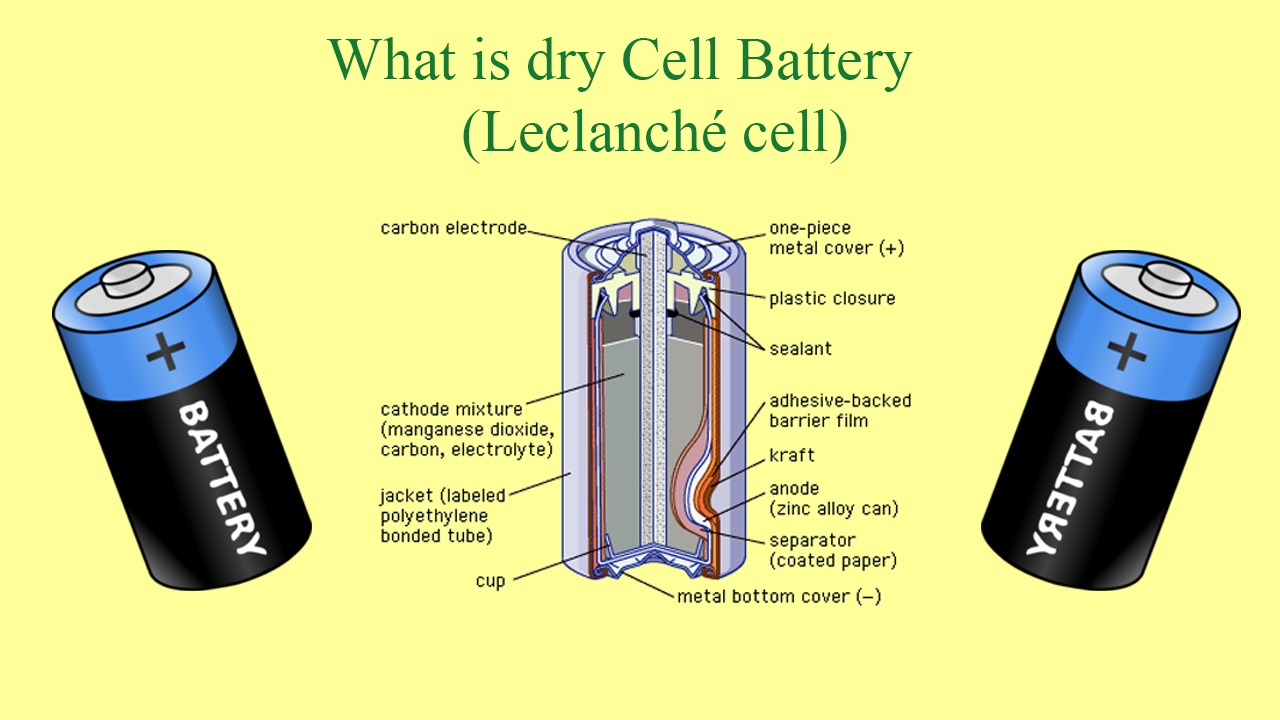
What is Dry Cell battery (Leclanché cell)? and main parts of dry cell battery
Images related to the topicWhat is Dry Cell battery (Leclanché cell)? and main parts of dry cell battery
What is ammonium chloride used for?
Its principal uses are as a nitrogen supply in fertilizers and as an electrolyte in dry cells, and it is also extensively employed as a constituent of galvanizing, tinning, and soldering fluxes to remove oxide coatings from metals and thereby improve the adhesion of the solders.
Why ammonium chloride is electrolyte?
Dear student, ammonium chloride is a salt formed by the combination of ammonium hydroxide, a weak base and hydrochloric acid, a strong acid. when it is dissolved in water will it ionise more and highly dissociate. It is also a good conductor of electricity. therefore it is a strong electrolyte.
Related searches to Which element is used in dry cells?
- uses of dry cell
- dry cell was invented by
- dry cell is also known as
- which element is used in dry cells at work
- what is dry cell
- a dry cell has how many terminals
- a dry cell has
- which element is used in dry cells in the body
- dry cell diagram
- dry cell is an example of
- which element is used in dry cells and storage batteries
Information related to the topic Which element is used in dry cells?
Here are the search results of the thread Which element is used in dry cells? from Bing. You can read more if you want.
You have just come across an article on the topic Which element is used in dry cells?. If you found this article useful, please share it. Thank you very much.