Are you looking for an answer to the topic “Which element is oxidized in a redox reaction?“? We answer all your questions at the website Ecurrencythailand.com in category: +15 Marketing Blog Post Ideas And Topics For You. You will find the answer right below.
An atom is oxidized if its oxidation number increases, the reducing agent, and an atom is reduced if its oxidation number decreases, the oxidizing agent. The atom that is oxidized is the reducing agent, and the atom that is reduced is the oxidizing agent.Oxidation–reduction reactions, commonly known as redox reactions, are reactions that involve the transfer of electrons from one species to another. The species that loses electrons is said to be oxidized, while the species that gains electrons is said to be reduced.

What is oxidized in a redox reaction?
Oxidation–reduction reactions, commonly known as redox reactions, are reactions that involve the transfer of electrons from one species to another. The species that loses electrons is said to be oxidized, while the species that gains electrons is said to be reduced.
Is oxygen oxidized or reduced?
Oxygen is therefore an oxidizing agent. Oxidizing and reducing agents therefore can be defined as follows. Oxidizing agents gain electrons. Reducing agents lose electrons.
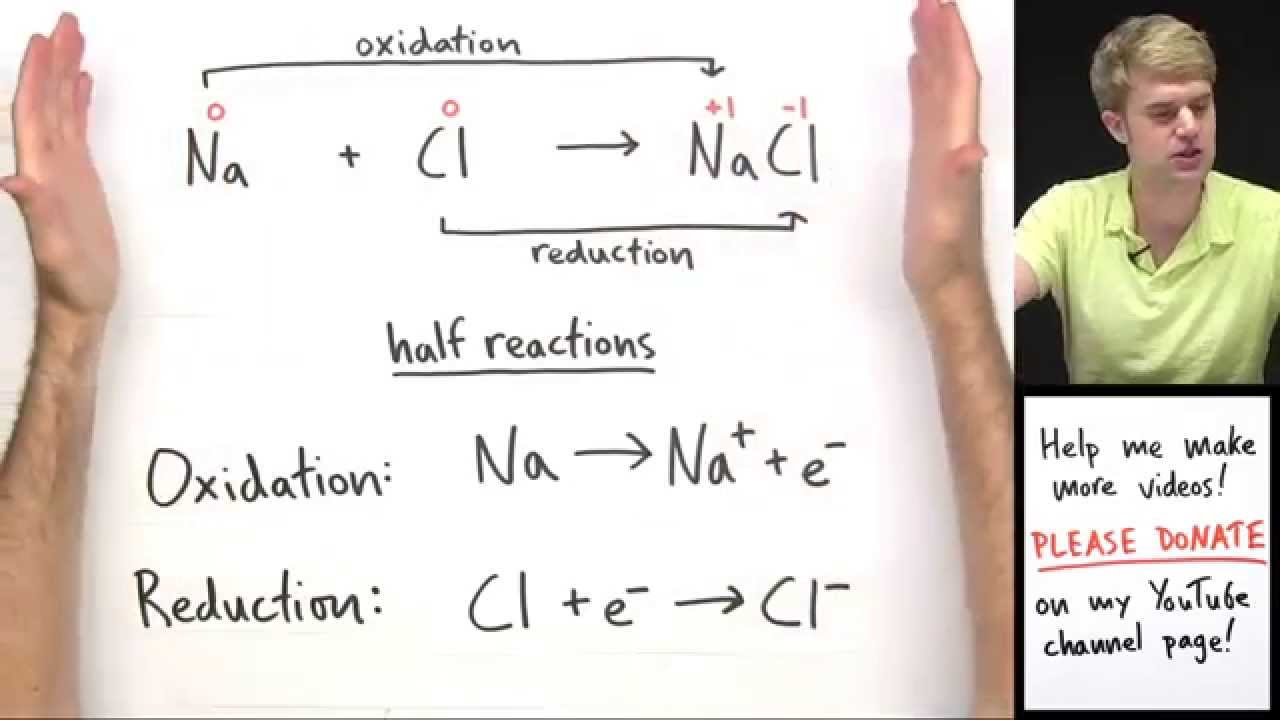
Oxidation and Reduction Reactions – Basic Introduction
Images related to the topicOxidation and Reduction Reactions – Basic Introduction

Is NADH oxidized or reduced?
Electron Carriers
Nicotinamide adenine dinucleotide (NAD) (Figure 4.13) is derived from vitamin B3, niacin. NAD+ is the oxidized form of the molecule; NADH is the reduced form of the molecule after it has accepted two electrons and a proton (which together are the equivalent of a hydrogen atom with an extra electron).
What are the examples of redox reaction?
…
Examples of these types of reactions are:
- 2NaH → 2Na + H. …
- 2H2O → 2H2 + O. …
- Na2CO3 → Na2O + CO.
Which element is oxidized and which is reduced in N2 g )+ 3H2 G → 2NH3 G?
The equation for the reaction is: N2(g) + 3H2(g) + 2NH3(g) Page 4 CHEMICAL REACTIONS 69 SOLUTION: The oxidation number of nitrogen changes from 0 to -3. The N2 is the oxidizing agent. The oxidizing number of hydrogen changes from 0 to +1.
What is redox reaction give an example?
An oxidation-reduction reaction is any chemical reaction in which, by obtaining or losing an electron, the oxidation number of a molecule, atom, or ion varies. An example of a redox reaction is the formation of hydrogen fluoride. To study the oxidation and reduction of reactants, we should break the reaction down.
Is NAD+ oxidized or reduced?
NAD can exist in two forms: NAD+ and NADH. These two forms of NAD are known as a “redox couple,” a term that is used to describe a reduced (the “red” in redox) and oxidized (the “ox” in redox) form of the same atom or molecule. The term “oxidized” can be misleading, though, as it does not necessarily require oxygen.
See some more details on the topic Which element is oxidized in a redox reaction? here:
Oxidation–reduction (redox) reactions (article) | Khan Academy
An oxidation–reduction or redox reaction is a reaction that involves the transfer of electrons between chemical species (the atoms, ions, or molecules …
20.1: Oxidation States and Redox Reactions – Chemistry …
An oxidation-reduction reaction is any chemical reaction in which the oxidation number of a molecule, atom, or ion changes by gaining or …
Oxidation and Reduction
The term oxidation was originally used to describe reactions in which an element combines with oxygen.
Understanding Redox Reactions & Oxidation Reduction
Redox is a shorthand for reduction-oxidation, meaning that a redox reaction is one in which both a reduction reaction and an oxidation reaction …
What does it mean when an element is oxidized?
An atom is oxidized if its oxidation number increases, the reducing agent, and an atom is reduced if its oxidation number decreases, the oxidizing agent. The atom that is oxidized is the reducing agent, and the atom that is reduced is the oxidizing agent.
Introduction to Oxidation Reduction (Redox) Reactions
Images related to the topicIntroduction to Oxidation Reduction (Redox) Reactions
Which of the following is an example of oxidation?
Therefore, Sn+2−2e−→Sn+4 is an example of oxidation.
Is CL oxidized or reduced?
…
| Substance oxidized | Substance reduced |
|---|---|
| is the reducing agent | is the oxidizing agent |
What causes an element to be oxidized?
An oxidizing agent is a substance that causes oxidation by accepting electrons, and a reducing agent is a substance that causes reduction by losing electrons. Said another way, the oxidizing agent is the substance that is reduced, while the reducing agent is the substance that is oxidized.
Which elements are oxidized and reduced in the reaction br2?
Bromine (Br) is oxidized, and iodine (I) is reduced. Use the chemical equation to answer the question. Which elements are oxidized and reduced in the reaction? Sodium (Na) is oxidized, and bromine (Br) is reduced.
Is NAD+ The oxidized form?
The cofactor is, therefore, found in two forms in cells: NAD+ is an oxidizing agent – it accepts electrons from other molecules and becomes reduced. This reaction, also with H+, forms NADH, which can then be used as a reducing agent to donate electrons. These electron transfer reactions are the main function of NAD.
Is glucose oxidized or reduced?
Glucose reacts with molecular oxygen to produce carbon dioxide and water. The carbon atoms in glucose are oxidized. That is, they lose electron and go to a higher oxidation state. The oxygen atoms in molecular oxygen are reduced.
Is NAD+ is in its oxidized form?
…
CHEBI:13389 – NAD.
| ChEBI Name | NAD |
|---|---|
| ChEBI ID | CHEBI:13389 |
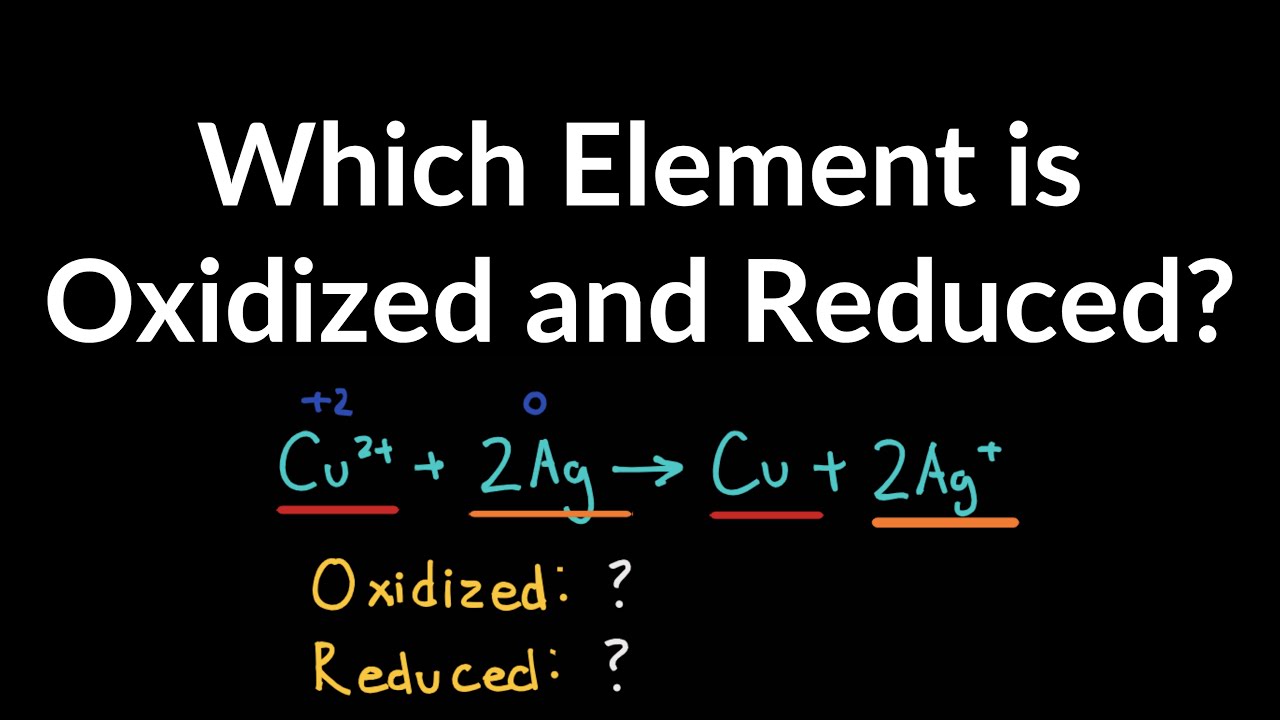
How to Identify Oxidized and Reduced Element in Redox Reaction with Examples and Problems
Images related to the topicHow to Identify Oxidized and Reduced Element in Redox Reaction with Examples and Problems
Which of the following is an oxidizing agent?
Common oxidizing agents are oxygen, hydrogen peroxide and the halogens.
What are the 3 types of redox reaction?
- Combination Reactions.
- Decomposition Reactions.
- Displacement Reactions.
- Disproportionate Reactions.
Related searches to Which element is oxidized in a redox reaction?
- how to identify a redox reaction
- as an element is oxidized its oxidation number
- 10 examples of redox reaction
- what is the element which has been oxidized in the redox reaction called
- how to tell if its redox reaction
- what is oxidized in a redox reaction
- what are the components that make up redox reaction
- oxidizing agent lowers the oxidation number of a given element
- what does it mean when an element is oxidized
- in an oxidationreduction reaction what happens to the electrons in the oxidation process
- which of the following is an oxidation reduction reaction
- which element is oxidized in a redox reaction
- as an element is oxidized, its oxidation number
- how to know which element is oxidized in a reaction
- which element is oxidized
- what is oxidized in a reaction
Information related to the topic Which element is oxidized in a redox reaction?
Here are the search results of the thread Which element is oxidized in a redox reaction? from Bing. You can read more if you want.
You have just come across an article on the topic Which element is oxidized in a redox reaction?. If you found this article useful, please share it. Thank you very much.