Are you looking for an answer to the topic “What will be formed after oxidation of 2 degree alcohol with chromic acid?“? We answer all your questions at the website Ecurrencythailand.com in category: +15 Marketing Blog Post Ideas And Topics For You. You will find the answer right below.
Chromic acid is most commonly used to oxidize 2o alcohols to ketones.The chromic acid oxidizes secondary alcohol to a ketone. As shown in the below example it oxidizes isopropyl alcohol to acetone. Therefore, the correct option for the above answer is – option (D) ie Ketone.Alcohol Oxidation Reactions with Chromic Acid
Chromic Acid is the stronger of the two oxidizing agents. It will carry out both steps of oxidation of a primary alcohol producing a carboxylic acid as well as oxidizing secondary alcohols to ketones. It will also oxidize an aldehyde to a carboxylic acid.

What is formed after oxidation of 2 alcohol with chromic acid?
The chromic acid oxidizes secondary alcohol to a ketone. As shown in the below example it oxidizes isopropyl alcohol to acetone. Therefore, the correct option for the above answer is – option (D) ie Ketone.
Can you oxidize a secondary alcohol with chromic acid?
Alcohol Oxidation Reactions with Chromic Acid
Chromic Acid is the stronger of the two oxidizing agents. It will carry out both steps of oxidation of a primary alcohol producing a carboxylic acid as well as oxidizing secondary alcohols to ketones. It will also oxidize an aldehyde to a carboxylic acid.
Chromic acid oxidation of alcohols
Images related to the topicChromic acid oxidation of alcohols

What happens when 2 alcohol is oxidised?
The oxidation of alcohols is an important reaction in organic chemistry. Primary alcohols can be oxidized to form aldehydes and carboxylic acids; secondary alcohols can be oxidized to give ketones.
What does chromic acid do to secondary alcohols?
Chromic acid oxidizes primary alcohols to carboxylic acids, and it oxidizes secondary alcohols to ketones.
What results are expected when the chromic acid test is done on primary alcohol?
The reaction of the Jonas reactant with a primary alcohol is given by the equation: A change in the solution’s color from red orange (chromic acid) to blue green (Cr(III)) ion indicates a positive result.
What is the formula of chromic acid?
Which alcohol will not be oxidized by chromic acid?
Tertiary alcohols do not react with chromic acid under mild conditions. With a higher temperature or a more concentrated, carbon-carbon bonds may be oxidized; however, yields from such strong oxidations are usually poor.
See some more details on the topic What will be formed after oxidation of 2 degree alcohol with chromic acid? here:
Reactions of alcohols – Encyclopedia Britannica
Alcohols may be oxidized to give ketones, aldehydes, and carboxylic acids. … Tertiary alcohols do not react with chromic acid under mild conditions.
The Oxidation of Alcohols – ChemistryViews
The oxidation of alcohols is an important reaction in organic chemistry. Primary alcohols can be oxidized to form aldehydes and carboxylic …
19.6. Oxidation of alcohols & aldehydes | Organic Chemistry II
A common method for oxidizing secondary alcohols to ketones uses chromic acid (H2CrO4) as the oxidizing agent. Chromic acid, also known as Jones reagent, is …
4. Oxidation Reactions of Alcohols – MSU chemistry
The most generally useful reagents for oxidizing 1º and 2º-alcohols are chromic acid derivatives. Two such oxidants are Jones reagent (a solution of sodium …
What products are formed when an alcohol is oxidized?
On of the most important reactions of alcohols is their oxidation to carbonyl containing compounds such as aldehyde, ketones, and carboxylic acid. Typically primary alcohols, depending on the reagent used, produce aldehydes or carboxylic acids during oxidations.
What is the chromic acid oxidation?
Mechanism of Oxidation by Chromium(VI) Chromic acid (H2CrO4) oxidizes alcohols in aqueous solutions of sodium dichromate. It reacts with alcohols to form a chromic ester in which the alcohol oxygen atom bridges the carbon and chromium atoms.
Which of the following alcohols is oxidised to a ketone by chromic acid?
Chromic acid oxidizes primary alcohols to carboxylic acids, and it oxidizes secondary alcohols to ketones. Tertiary alcohols do not react with chromic acid under mild conditions.
How do you turn alcohol into an aldehyde?
The primary alcohol is converted to aldehyde by the oxidation reaction using mild oxidizing reagent.
How are secondary alcohols converted to ketones?
3. Making of Ketones. The preparation of Ketones is done by the oxidation of secondary alcohols. Consider, for example, heating the secondary alcohol propan-2-ol with the sodium or even potassium dichromate(VI) solution which is acidified with the dilute sulphuric acid, then the ketone called propanone formed.
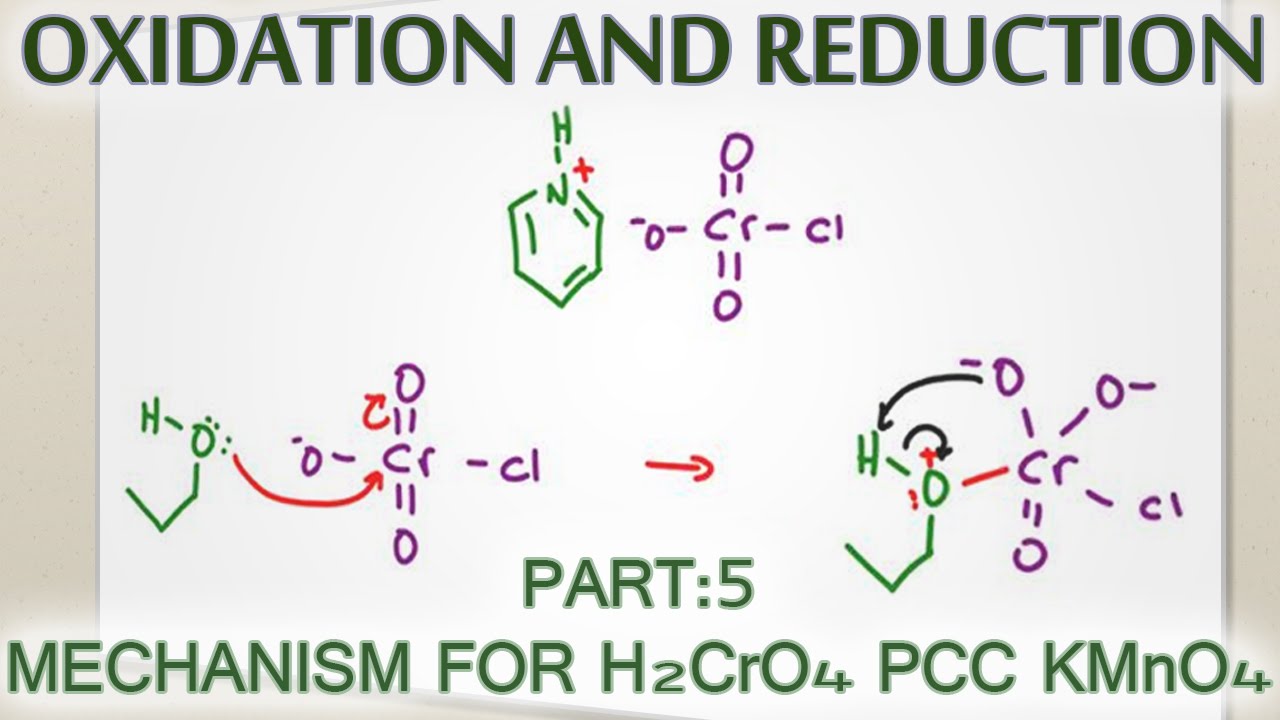
Alcohol Oxidation Mechanism with H2CrO4, PCC and KMnO4
Images related to the topicAlcohol Oxidation Mechanism with H2CrO4, PCC and KMnO4
What gives a positive result in the chromic acid test?
Three drops of the compound to be tested are mixed with 5 drops of acetone and 5 drops of chromic acid solution (an orange solution). Indications of a positive test: The disappearance of the red-orange color of chromic acid and the formation of a blue-green color of the Cr (III) ion indicates a positive test.
How do you make an alkene from an alcohol?
The dehydration reaction of alcohols to generate alkene proceeds by heating the alcohols in the presence of a strong acid, such as sulfuric or phosphoric acid, at high temperatures.
Which product is obtained by oxidation of phenol with chromic acid?
In the question, phenol is given as the starting material, but in the options, the product is either dione or diol. The product suggests that the starting material is hydroquinone. So instead of phenol, you start the reaction with hydroquinone. In presence of chromic acid, hydroquinone is oxidized to benzoquinone.
How will you distinguish primary secondary and tertiary alcohol by Lucas test?
Solution : Lucas test is used to differentiate between primary, secondary and tertiary alcohol. Lucas reagent consists of equimolar mixture of con. HCl and anhydrous `ZnCl_2` <br> If turbidity appears immediately alcohols is tertiary. If turbidity appears in about five minutes the alcohols is secondary.
What kind of alcohol gives positive chromic acid test?
Test 1: Chromic Acid Oxidation
This test distinguishes primary and secondary alcohols from tertiary. Chromic acid will oxidize a primary alcohol first to an aldehyde and then to a carboxylic acid and it will oxidize a secondary alcohol to a ketone. Tertiary alcohols do not react.
Is chromic acid an oxidizing agent?
Chromic acid is a strong oxidizing agent, used to oxidize many classes of organic compounds, the most common of which is alcohols.
What is oxidation number of Cr in chromic acid?
Chromium forms several commercially valuable oxygen compounds, the most important of which is chromium oxide, commonly called chromium trioxide or chromic acid, CrO3, in which chromium is in the +6 oxidation state.
What is the correct chemical name for cr2o3?
| PubChem CID | 517277 |
|---|---|
| Synonyms | Chromium(III) oxide 1308-38-9 Dichromium trioxide Chromia Chromium sesquioxide More… |
| Molecular Weight | 151.990 |
| Dates | Modify 2022-05-21 Create 2005-03-27 |
| Description | Dichromium trioxide is a chromium oxide. ChEBI |
What is the PH of chromic acid?
| Acid | Name | 100 mM |
|---|---|---|
| H2CrO4 | chromic acid | 2.06 |
| H3Citrate | citric acid, C6H8O7 | 2.08 |
| HF | hydrofluoric acid | 2.12 |
| HNO2 | nitrous acid | 2.13 |
Why are tertiary alcohols not oxidized?
Tertiary alcohols (R3COH) are resistant to oxidation because the carbon atom that carries the OH group does not have a hydrogen atom attached but is instead bonded to other carbon atoms. The oxidation reactions we have described involve the formation of a carbon-to-oxygen double bond.
12.7 Oxidation with Chromic Acid and PCC
Images related to the topic12.7 Oxidation with Chromic Acid and PCC

How do you make tertiary alcohol?
To produce a primary alcohol, the Grignard reagent is reacted with formaldehyde. Reacting a Grignard reagent with any other aldehyde will lead to a secondary alcohol. Finally, reacting a Grignard reagent with a ketone will generate a tertiary alcohol.
What products are formed when an alcohol undergoes dehydration?
Alcohols can be dehydrated to form either alkenes (higher temperature, excess acid) or ethers (lower temperature, excess alcohol). Primary alcohols are oxidized to form aldehydes.
Related searches to What will be formed after oxidation of 2 degree alcohol with chromic acid?
- chromic acid test for alcohols
- oxidation of alcohol with k2cr2o7 mechanism
- oxidation of secondary alcohol
- oxidation of alcohol with kmno4
- chromic acid oxidation
- oxidation of tertiary alcohol with kmno4
- what will be formed after oxidation of 2 degree alcohol with chromic acid
- oxidation of alcohols
- which of the following alcohols will give a positive chromic acid test
Information related to the topic What will be formed after oxidation of 2 degree alcohol with chromic acid?
Here are the search results of the thread What will be formed after oxidation of 2 degree alcohol with chromic acid? from Bing. You can read more if you want.
You have just come across an article on the topic What will be formed after oxidation of 2 degree alcohol with chromic acid?. If you found this article useful, please share it. Thank you very much.