Are you looking for an answer to the topic “Can a solution be Hyperosmotic and isotonic?“? We answer all your questions at the website Ecurrencythailand.com in category: +15 Marketing Blog Post Ideas And Topics For You. You will find the answer right below.
Non-penetrating solutes cannot cross the cell membrane; therefore, the movement of water across the cell membrane (i.e., osmosis) must occur for the solutions to reach equilibrium. A solution can be both hyperosmotic and isotonic.If a cell is in a hypertonic solution, the solution has a lower water concentration than the cell cytosol, and water moves out of the cell until both solutions are isotonic. Cells placed in a hypotonic solution will take in water across their membranes until both the external solution and the cytosol are isotonic.When a cell is placed in a hyperosmotic but hypotonic solution like 10% dextran, water movement will occur. Therefore, a solution can be hyperosmotic and hypotonic.

Can a solution be hypertonic and isotonic?
If a cell is in a hypertonic solution, the solution has a lower water concentration than the cell cytosol, and water moves out of the cell until both solutions are isotonic. Cells placed in a hypotonic solution will take in water across their membranes until both the external solution and the cytosol are isotonic.
Can a solution be Hyperosmotic and hypotonic?
When a cell is placed in a hyperosmotic but hypotonic solution like 10% dextran, water movement will occur. Therefore, a solution can be hyperosmotic and hypotonic.
Hypertonic, Hypotonic and Isotonic Solutions!
Images related to the topicHypertonic, Hypotonic and Isotonic Solutions!

What makes a solution Hyperosmotic?
Hyperosmotic can refer to solutions that have increased osmotic pressure, or a greater difference between solutes and solutions between a membrane. In other instances, hyperosmotic refers to a solution that has more solutes, or components of a solution, than a similar solution.
How can a solution be Isosmotic but not isotonic?
The difference between isotonic and isosmotic is that isotonic solutions contain only non-penetrating solutes whereas isosmotic solutions contain both penetrating as well as non-penetrating solutes.
How do you know if a solution is isotonic hypotonic or hypertonic?
Isotonic: The solutions being compared have equal concentration of solutes. Hypertonic: The solution with the higher concentration of solutes. Hypotonic: The solution with the lower concentration of solutes.
What are the three types of solution?
There are three types of solutions that can occur in your body based on solute concentration: isotonic, hypotonic, and hypertonic.
Is Hypoosmotic and hypotonic same?
Hyperosmotic solutions are not always hypertonic. But hyposmotic solutions are always hypotonic. The response to this rapid fire presentation of osmolarity and tonicity was overwhelmingly positive.
See some more details on the topic Can a solution be Hyperosmotic and isotonic? here:
Isosmotic is not always isotonic: the five-minute version
How can a hyperosmotic solution be hypotonic? Tonicity depends only on the concentration of nonpenetrating solutes, so any solution of pure …
Tonicity: hypertonic, isotonic & hypotonic solutions (article)
Three terms—hyerptonic, hypotonic, and isotonic—are used to describe whether a solution will cause water to move into or out of a cell:.
Hyperosmotic Definition and Examples – Biology Online
A classical example of a hypotonic solution is a 5% dextrose solution having no non-penetrating solutes. When a cell is placed in a hyperosmotic …
Tonicity And Osmolarity In The Blood – BYU-Idaho
He explains the differences between hyposmotic, hyperosmotic, isosmotic, hypotonic, hypertonic, and isotonic solutions. The tutor also tests …
Is it possible for a solution to be both hypertonic and hypotonic Why or why not?
Is it possible for a solution to be both hypertonic and hypotonic? Why or why not? Yes.
What is the difference between Hyperosmotic and Hypoosmotic?
The key difference between isosmotic hyperosmotic and hypoosmotic is that isosmotic refers to the property of having equal osmotic pressures. But, hyperosmotic refers to the property of having a high osmotic pressure and hypoosmotic refers to the property of having a low osmotic pressure.
Are hypertonic and Hyperosmotic the same?
Hyperosmotic solutions are not always hypertonic. But hyposmotic solutions are always hypotonic. The response to this rapid fire presentation of osmolarity and tonicity was overwhelmingly positive.
Is 0.9% NaCl hypertonic or hypotonic?
Hypertonic Solution
If a cell with a NaCl concentration of 0.9% is placed in a solution of water with a 10% concentration of NaCl, the solution is said to be hypertonic.
Which solution is Hyperosmotic to the other?
| • | If cell volume at equilibrium has increased, the solution is said to be hypotonic to the cell. |
|---|---|
| • | If cell volume at equilibrium has decreased, the solution is said to be hypertonic to the cell. |
| • | If cell volume at equilibrium has not changed, the solution is said to be isotonic to the cell. |
LPA 1B – Osmolarity vs Tonicity
Images related to the topicLPA 1B – Osmolarity vs Tonicity

What is the difference between isotonic and Isosmotic?
Isotonic refers to a solution having the same solute concentration as in a cell or a body fluid. Isosmotic refers to the situation of two solutions having the same osmotic pressure. Isosmotic solutions cause cells to absorb water from surrounding or to lose water from cells.
Are osmolarity and osmolality the same?
Osmolarity and osmolality are frequently confused and incorrectly interchanged. Osmolarity refers to the number of solute particles per 1 L of solvent, whereas osmolality is the number of solute particles in 1 kg of solvent.
What is meant by Isosmotic solution?
Definitions of isosmotic solution. a solution having the same osmotic pressure as blood. synonyms: isotonic solution.
What would make a hypertonic solution isotonic?
An example of a hypertonic solution is the interior of a red blood cell compared with the solute concentration of fresh water. When two solutions are in contact, solute or solvent moves until the solutions reach equilibrium and become isotonic with respect to each other.
What are hypertonic solutions?
Hypertonic solution: A solution that contains more dissolved particles (such as salt and other electrolytes) than is found in normal cells and blood. For example, hypertonic solutions are used for soaking wounds.
What are some examples of isotonic hypertonic and hypotonic solutions?
- Hypertonic: D5 NaCl. D5 in Lactated ringers. D5 0.45% NaCl.
- Isotonic: 0.9% NaCl (Normal Saline) Lactated Ringers. D5W (In the bag)
- Hypotonic: D5W (in the body) 0.25% NaCl. 0.45% NaCl (half normal saline) 2.5% Dextrose.
What are the two classifications of solutions?
Aqueous solution – When a solute is dissolved in water the solution is called an aqueous solution. Eg, salt in water, sugar in water and copper sulfate in water. Non-aqueous solution – When a solute is dissolved in a solvent other than water, it is called a non-aqueous solution.
How can a solution be both dilute and saturated?
A solution can be both saturated and dilute. For example, consider solution of sparingly soluble salt in water. No more solute can be dissolved in such a solution. Hence, it is a saturated solution.
What is homogeneous and heterogeneous solution?
A homogenous mixture is that mixture in which the components mix with each other and its composition is uniform throughout the solution. A heterogenous mixture is that mixture in which the composition is not uniform throughout and different components are observed.
What does it mean to be Hypoosmotic?
adjective. 1. Of, relating to, or characterized by having a lower osmotic pressure than a surrounding fluid under comparison. 2. A condition in which the total amount of solutes (both permeable and impermeable) in a solution is lower than that of another solution.
Tonicity Osmolarity
Images related to the topicTonicity Osmolarity
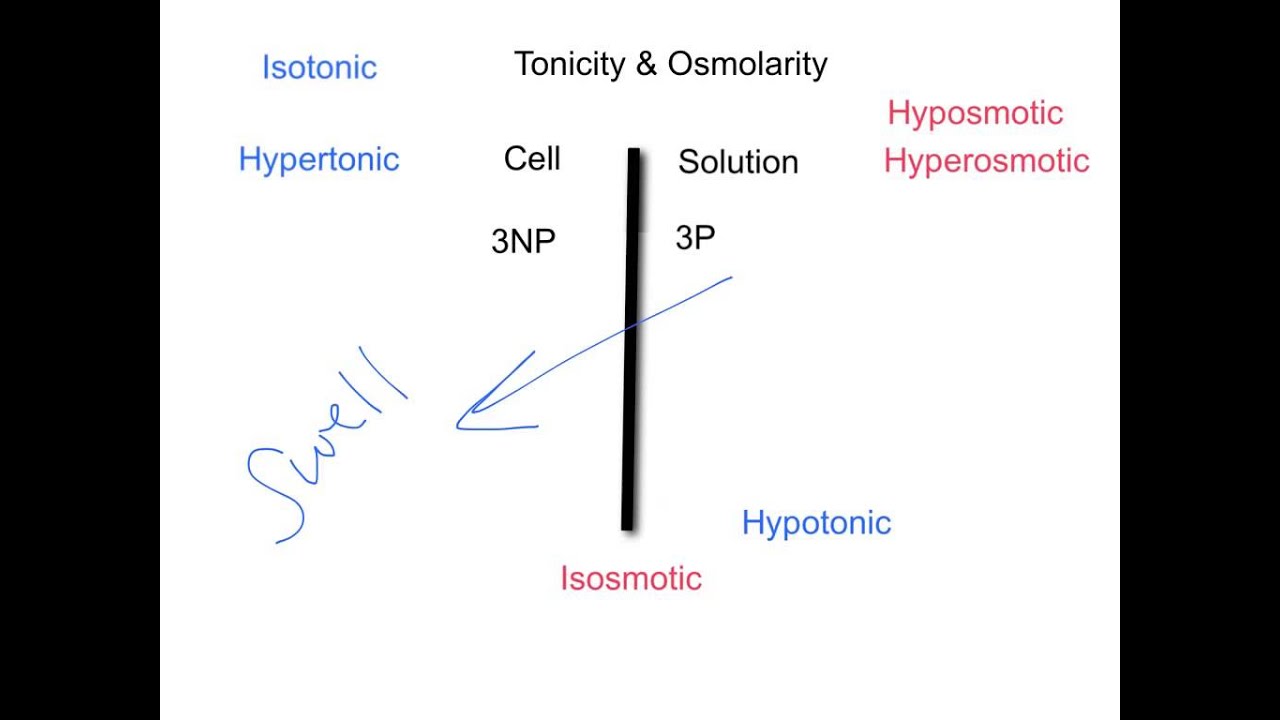
What is the difference between osmolality and tonicity?
Osmolality is a property of a particular solution and is independent of any membrane. Tonicity is a property of a solution in reference to a particular membrane.
What happens to a cell in a Hypoosmotic solution?
Under these conditions, the osmotic pressure gradient forces water into the cell. Depending on the amount of water that enters, the cell may look enlarged or bloated. If the water continues to move into the cell, it can stretch the cell membrane to the point the cell bursts (lyses) and dies.
Related searches to Can a solution be Hyperosmotic and isotonic?
- isotonic solution
- hypertonic solution
- why are hyperosmotic solutions always hypotonic
- how does isotonic solution treat dehydration
- isotonic hypertonic hypotonic
- hypotonic solution example
- why give isotonic solution for dehydration
- isotonic, hypertonic hypotonic
- difference between isotonic and isosmotic solution
- hypotonic solution
- can a hyperosmotic solution be hypotonic
- what happens when you drink isotonic solution
- hyperosmotic vs hypoosmotic
- what happens to a cell in a hypotonic solution
- can a solution be hyperosmotic and isotonic
Information related to the topic Can a solution be Hyperosmotic and isotonic?
Here are the search results of the thread Can a solution be Hyperosmotic and isotonic? from Bing. You can read more if you want.
You have just come across an article on the topic Can a solution be Hyperosmotic and isotonic?. If you found this article useful, please share it. Thank you very much.