Are you looking for an answer to the topic “What is elementary reaction Give difference between order and Molecularity?“? We answer all your questions at the website Ecurrencythailand.com in category: +15 Marketing Blog Post Ideas And Topics For You. You will find the answer right below.
(a) Order of a reaction is the sum of the coefficients of the reacting species involved in the rate equation. (a) Molecularity is the number of reacting species involved in simultaneous collisions in an elementary or simplest reaction.Elementary (single-step) reactions and reaction steps have reaction orders equal to the stoichiometric coefficients for each reactant. The overall reaction order, i.e. the sum of stoichiometric coefficients of reactants, is always equal to the molecularity of the elementary reaction.Elementary reaction: It is defined as a chemical reaction in which one or more chemical species react directly with each other to form the product in a single step.
| Molecularity | Order |
|---|---|
| It represents the number of the reactant molecules taking part in the elementary reaction. | It represents the sum of the exponents to which the concentration term in the rate law are raised. |
| Order | Molecularity |
|---|---|
| Order might involve one elementary reaction or it might involve a sequence of elementary reactions. | Molecularity is a part of the whole reaction. |
| It is an experimentally determined value. | It is a theoretical concept. |
| It can have fractional value. | It is always a whole number. |
What is an elementary reaction Give the differences between order and molecularity of a reaction?
| Molecularity | Order |
|---|---|
| It represents the number of the reactant molecules taking part in the elementary reaction. | It represents the sum of the exponents to which the concentration term in the rate law are raised. |
What is elementary reaction order?
Elementary (single-step) reactions and reaction steps have reaction orders equal to the stoichiometric coefficients for each reactant. The overall reaction order, i.e. the sum of stoichiometric coefficients of reactants, is always equal to the molecularity of the elementary reaction.
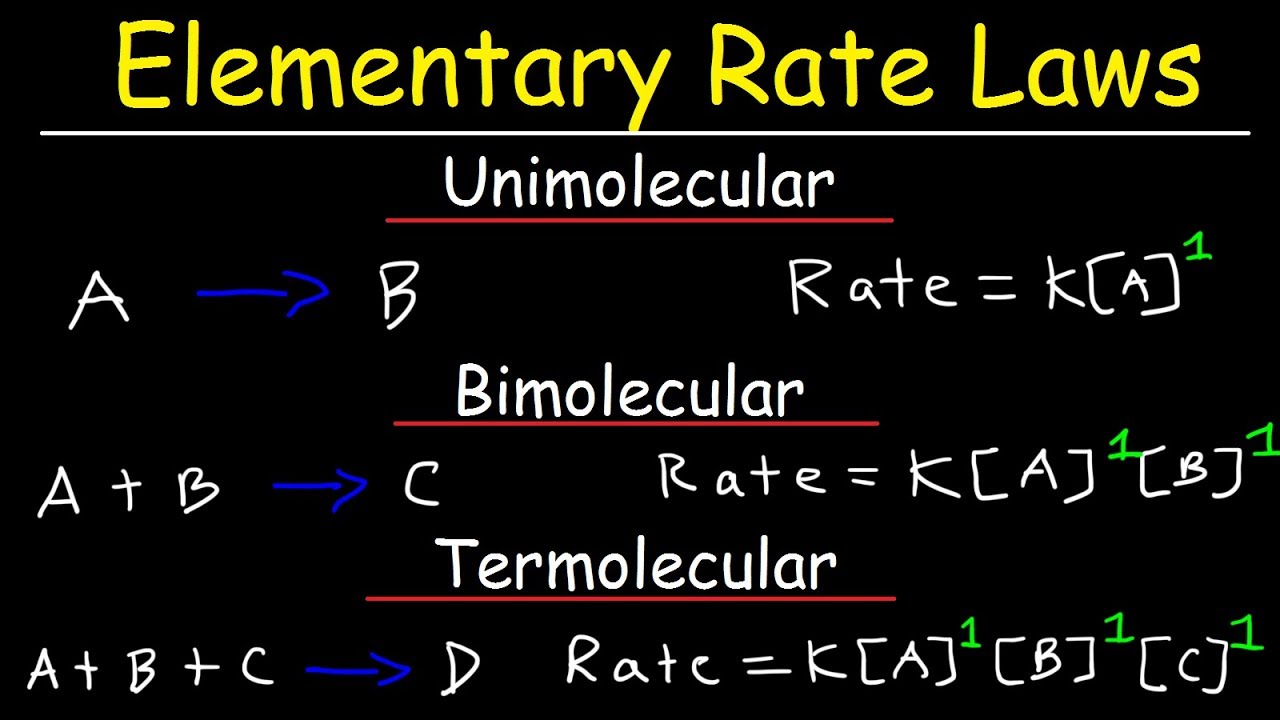
Elementary Rate Laws – Unimolecular, Bimolecular and Termolecular Reactions – Chemical Kinetics
Images related to the topicElementary Rate Laws – Unimolecular, Bimolecular and Termolecular Reactions – Chemical Kinetics

What is an elementary reaction Class 12th?
Elementary reaction: It is defined as a chemical reaction in which one or more chemical species react directly with each other to form the product in a single step.
What is the basic difference between order and molecularity?
| Order | Molecularity |
|---|---|
| Order might involve one elementary reaction or it might involve a sequence of elementary reactions. | Molecularity is a part of the whole reaction. |
| It is an experimentally determined value. | It is a theoretical concept. |
| It can have fractional value. | It is always a whole number. |
What difference between molecularity and order of a reaction write any four differences?
…
Chemical Kinetics.
| Order | Molecularity |
|---|---|
| It is determined experimentally. | It is a theoretical concept. |
| It may be equal to zero or have fractional values. | It cannot be equal to zero and it always has integral values( which cannot exceed 3) |
What is order and molecularity of reaction?
The order of reaction is an empirical quantity determined by experiment from the rate law of the reaction. It is the sum of the exponents in the rate law equation. Molecularity, on the other hand, is deduced from the mechanism of an elementary reaction, and is used only in context of an elementary reaction.
What is elementary reaction example?
An elementary reaction is a reaction that occurs in a single step. The rate law for an elementary reaction can be derived from the coefficients of the reactants in the balanced equation. For example, the rate law for the elementary reaction 2A + B → products is rate = k[A]²[B].
See some more details on the topic What is elementary reaction Give difference between order and Molecularity? here:
Why are molecularity and order the same in an elementary …
Molecularity is number of molecules participate in a reaction. · Order is the experimental quantity. On the other way order is the no of molecules which …
Write the difference between order and molecularity of reaction.
For a simple chemical reaction that occurs in one step, the molecularity tells us how many molecules affect the rate of that reaction. And order tells about …
Write four differences between molecularity and order of …
Molecularity, Order. It represents the number of the reactant molecules taking part in the elementary reaction. It represents the sum of the exponents to …
Distinguish Between Molecularity and Order of Reaction.
2. It is the sum of powers of the concentration terms of reactants that appear in the rate equation. It is the number of reactant molecules taking part in an …
What is an elementary process?
elementary process in American English
noun. Physical Chemistry. a chemical process complete in one step, characterized by the simultaneous interaction of all the atoms of two or more molecules.
What is molecularity of a reaction explain its types by examples?
The molecularity of a reaction is defined as the number of reacting molecules which collide simultaneously to bring about a chemical reaction. In other words, the molecularity of an elementary reaction is defined as the number of reactant molecules taking part in the reaction. For example, consider the reaction.
Order vs. Molecularity
Images related to the topicOrder vs. Molecularity

What is elementary reaction answer?
An elementary reaction is a chemical reaction in which one or more chemical species react directly to form products in a single reaction step and with a single transition state.
What is elementary reaction shaala?
Solution. Each and every single step in a reaction mechanism is called an elementary reaction.
What is elementary state in chemistry?
An elementary reaction is a chemical reaction where reactants form products in a single step with a single transition state. Elementary reactions may combine to form complex or nonelementary reactions.
What are two differences between order and molecularity?
…
Solution 1.
| ORDER OF A REACTION | MOLECULARITY OF A REACTION |
|---|---|
| It need not be a whole number i.e. it can be fractional as well as zero. | It is always a whole number. |
What is order of a reaction?
Definition. The Order of Reaction refers to the power dependence of the rate on the concentration of each reactant. Thus, for a first-order reaction, the rate is dependent on the concentration of a single species.
What is the order of reaction Class 12?
The order of a reaction is defined as: the sum of the powers to which the concentration terms are raised in the rate law equation to express the observed rate of the reaction. The power of the concentration of a particular reactant in the rate law is called the order of the reaction with respect to that reactant.
What is first order reaction?
Definition of first-order reaction
: a chemical reaction in which the rate of reaction is directly proportional to the concentration of the reacting substance — compare order of a reaction.
Difference between Order and Molecularity |ENGLISH|
Images related to the topicDifference between Order and Molecularity |ENGLISH|

What is the molecularity of each of the elementary steps?
Both elementary steps are bi-molecular as in each elementary step, two reactant molecules participate to form products. In other words, the molecularity of each of the elementary steps is two.
For which type of reaction order and molecularity have the same value a elementary reaction B complex reaction C both A & B d none of these?
Elementary reactions have same value of order and molecularity.
Related searches to What is elementary reaction Give difference between order and Molecularity?
- 10 differences between molecularity and order of reaction
- difference between order and molecularity ppt
- difference between rate of reaction and rate constant
- order of reaction
- order and molecularity of reaction
- molecularity and order of reaction pdf
- similarities between order and molecularity
Information related to the topic What is elementary reaction Give difference between order and Molecularity?
Here are the search results of the thread What is elementary reaction Give difference between order and Molecularity? from Bing. You can read more if you want.
You have just come across an article on the topic What is elementary reaction Give difference between order and Molecularity?. If you found this article useful, please share it. Thank you very much.