Are you looking for an answer to the topic “What is Erwin Schrodinger atomic theory?“? We answer all your questions at the website Ecurrencythailand.com in category: +15 Marketing Blog Post Ideas And Topics For You. You will find the answer right below.
Based on de Broglie’s idea that particles could exhibit wavelike behavior, Austrian physicist Erwin Schrödinger theorized that the behavior of electrons within atoms could be explained by treating them mathematically as matter waves.Assuming that matter (e.g., electrons) could be regarded as both particles and waves, in 1926 Erwin Schrödinger formulated a wave equation that accurately calculated the energy levels of electrons in atoms.Erwin Schrodinger. A powerful model of the atom was developed by Erwin Schrödinger in 1926. Schrödinger combined the equations for the behavior of waves with the de Broglie equation to generate a mathematical model for the distribution of electrons in an atom.

How did Erwin Schrödinger contribute to the atomic theory?
Assuming that matter (e.g., electrons) could be regarded as both particles and waves, in 1926 Erwin Schrödinger formulated a wave equation that accurately calculated the energy levels of electrons in atoms.
When did Erwin Schrödinger contribute to the atomic theory?
Erwin Schrodinger. A powerful model of the atom was developed by Erwin Schrödinger in 1926. Schrödinger combined the equations for the behavior of waves with the de Broglie equation to generate a mathematical model for the distribution of electrons in an atom.
Schrodinger’s Model
Images related to the topicSchrodinger’s Model

Who is Erwin Schrödinger and what did he do?
Austrian physicist Erwin Schrödinger was a noted theoretical physicist and scholar who came up with a groundbreaking wave equation for electron movements. He was awarded the 1933 Nobel Prize in Physics, along with British physicist P.A.M. Dirac, and later became a director at Ireland’s Institute for Advanced Studies.
What is Schrödinger’s theory called?
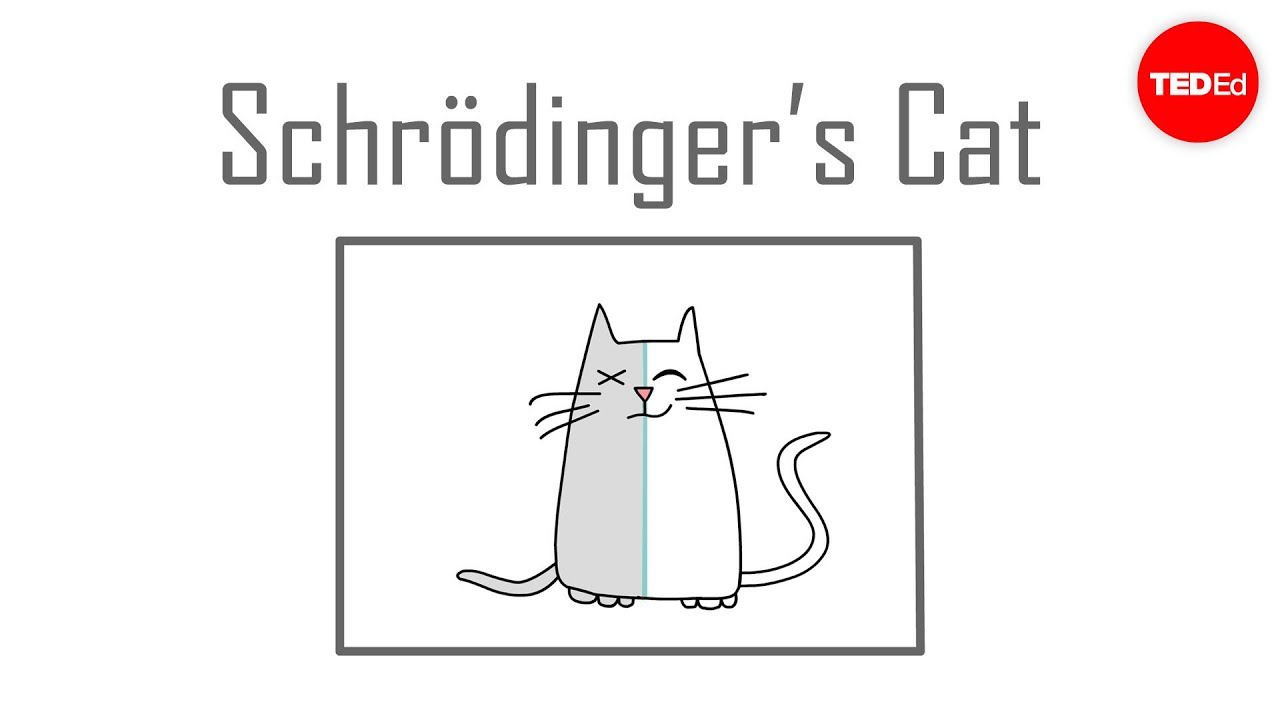
In quantum mechanics, Schrödinger’s cat is a thought experiment that illustrates a paradox of quantum superposition. In the thought experiment, a hypothetical cat may be considered simultaneously both alive and dead as a result of its fate being linked to a random subatomic event that may or may not occur.
Why was Erwin Schrödinger important?
He made notable contributions in quantum physics, wave mechanics and unified filed theory. He was awarded the Nobel Prize in Physics in 1933 for his work in the field of quantum mechanics and establishing the Schrodinger equation, which provides a way to calculate the wave function of a system.
Schrödinger Equation : its impact on the electron and the atom
Images related to the topicSchrödinger Equation : its impact on the electron and the atom

When did Erwin Schrödinger make his discovery?
His great discovery, Schrödinger’s wave equation, was made at the end of this epoch-during the first half of 1926. It came as a result of his dissatisfaction with the quantum condition in Bohr’s orbit theory and his belief that atomic spectra should really be determined by some kind of eigenvalue problem.
See some more details on the topic What is Erwin Schrodinger atomic theory? here:
Erwin Schrödinger – Facts – NobelPrize.org
In Niels Bohr’s theory of the atom, electrons absorb and emit radiation of fixed wavelengths when jumping between fixed orbits around a nucleus. The theory …
Development of the Atomic Theory
In 1926 Erwin Schrödinger, an Austrian physicist, took the Bohr atom model one step further. Schrödinger used mathematical equations to describe the …
Erwin Schrodinger
A powerful model of the atom was developed by Erwin Schrödinger in 1926. Schrödinger combined the equations for the behavior of waves with the de Broglie …
The History of the Atomic Model: Schrodinger and the Wave …
Schrodinger’s theory made use of electrons as waves, treating them as clouds of negative charge. Schrodinger’s equation mathematically determined the regions …
What is life Erwin Schrödinger quotes?
- “Consciousness cannot be accounted for in physical terms. …
- “If a man never contradicts himself, the reason must be that he virtually never says anything at all.” …
- “The scientist only imposes two things, namely truth and sincerity, imposes them upon himself and upon other scientists.”
How can the cat be both dead and alive?
“Schrodinger’s cat is not both dead and alive any more than an electron simultaneously exists at every point in space. ” and “Therefore, the cat is in a state that is a superposition of our “life states” |alive⟩ and |dead⟩”.
How did Schrödinger discover the electron cloud?
Schrödinger developed an equation that could be used to calculate the chances of an electron being in any given place around the nucleus. Based on his calculations, he identified regions around the nucleus where electrons are most likely to be. He called these regions orbitals.
Schrödinger’s cat: A thought experiment in quantum mechanics – Chad Orzel
Images related to the topicSchrödinger’s cat: A thought experiment in quantum mechanics – Chad Orzel
How does the living organism avoid decay?
How does the living organism avoid decay? The obvious answer is: By eating, drinking, breathing and (in the case of plants) assimilating. The technical term is metabolism.
What is it that called you suddenly out of nothingness?
“What is it that has called you so suddenly out of nothingness to enjoy for a brief while a spectacle which remains quite indifferent to you? The conditions for your existence are almost as old as the rocks. For thousands of years men have striven and suffered and begotten and women have brought forth in pain.
Related searches to What is Erwin Schrodinger atomic theory?
- erwin schrodinger atomic theory date
- erwin schrödinger interesting facts
- erwin schrodinger atomic theory experiment
- james chadwick atomic model
- erwin schrödinger atomic theory date
- what was erwin schrodinger atomic model
- erwin schrodinger interesting facts
- heisenberg atomic model
- what did erwin schrodinger do for the atomic theory
- erwin schrodinger cat
- what was erwin schrodinger contribution to the atomic theory
- what is erwin schrodinger atomic theory
- what did erwin schrodinger discover
- what did erwin schrödinger discover
- modern atomic model
Information related to the topic What is Erwin Schrodinger atomic theory?
Here are the search results of the thread What is Erwin Schrodinger atomic theory? from Bing. You can read more if you want.
You have just come across an article on the topic What is Erwin Schrodinger atomic theory?. If you found this article useful, please share it. Thank you very much.