Are you looking for an answer to the topic “What is extraction solvent ethanol?“? We answer all your questions at the website Ecurrencythailand.com in category: +15 Marketing Blog Post Ideas And Topics For You. You will find the answer right below.
Ethanol Extractions is a process used in fine liquor distillation. It is done by soaking raw cannabis in ethanol to pull out a solvent and the cannabis is then removed. The ethanol extraction process is used to filter out alcohol content from extracted material.Ethanol used in the extraction processes is usually USP grade. This stands for United States Pharmacopeia and means the product is acceptable for food, drug, or medicinal use. Companies can also choose to use denatured ethanol. This type of ethanol has a component that is poisonous and is not suitable for consumption.Ethanol has been known as a good solvent for polyphenol extraction and is safe for human consumption. Methanol has been generally found to be more efficient in extraction of lower molecular weight polyphenols, whereas aqueous acetone is good for extraction of higher molecular weight flavanols [6].

What type of ethanol is used for extraction?
Ethanol used in the extraction processes is usually USP grade. This stands for United States Pharmacopeia and means the product is acceptable for food, drug, or medicinal use. Companies can also choose to use denatured ethanol. This type of ethanol has a component that is poisonous and is not suitable for consumption.
Can you use ethanol as extraction solvent?
Ethanol has been known as a good solvent for polyphenol extraction and is safe for human consumption. Methanol has been generally found to be more efficient in extraction of lower molecular weight polyphenols, whereas aqueous acetone is good for extraction of higher molecular weight flavanols [6].
Cannabis Extraction Explained: Ethanol vs. Supercritical CO2 vs Hydrocarbon Extraction
Images related to the topicCannabis Extraction Explained: Ethanol vs. Supercritical CO2 vs Hydrocarbon Extraction

What is solvent ethanol?
Ethanol is an important industrial chemical; it is used as a solvent, in the synthesis of other organic chemicals, and as an additive to automotive gasoline (forming a mixture known as a gasohol). Ethanol is also the intoxicating ingredient of many alcoholic beverages such as beer, wine, and distilled spirits.
Why ethanol is a good solvent?
Ethanol is considered a universal solvent, as its molecular structure allows for the dissolving of both polar, hydrophilic and nonpolar, hydrophobic compounds.
Is ethanol and ethyl alcohol the same?
Ethanol, also known as ethyl alcohol, C2H5OH, is a colorless flammable slightly toxic compound that is made in fermentation and is used as a solvent and as an antifreeze. Ethanol also known as ethyl alcohol because it is commonly known as the alcohol found in alcoholic beverages.
Why 80% ethanol is used in plant extraction?
When 80% ethanol was used for extraction the high- est amount of flavonoids was detected and also the best antioxidant capacity. Conclusions. The results clearly showed that utilization of polar solvent enable extraction of significant amounts of phenolics and flavonoids.
Why do we use ethanol in plant extraction?
Most importantly ethanol is both highly effective and perfectly safe to use for plant extraction. Ethanol will retrieve a large amount of oil from the plant and then completely evaporate. This results in the maximum amount of product from your plant that is safe to use in both food grade and consumable goods.
See some more details on the topic What is extraction solvent ethanol? here:
Solvent Extraction Method of Plants Using Ethanol – Cole …
Typically, high-proof alcohols (190 and 200) have been used for extraction applications. Ethanol is emerging as one of the more popular solvents …
What’s the Best Ethanol for Commercial Solvent Extraction …
Ethanol (EtOH or ethyl alcohol) is a multifaceted solvent that mixes without separation in water and other organic solvents including acetic …
Pros & Cons of Ethanol Extraction
With hydrocarbon, the solvent is non-polar, meaning it binds to the more fat soluble components of the plant (cannabinoids and terpenes only).
Solvent Extraction of Ethanol from Aqueous Solutions. I …
Distillation to near the azeotrope, followed by pressure-swing adsorption for final dehydration, is the predominant method in the United States …
What is the best solvent for extraction?
Methanol was identified as the most effective solvent for the extraction, resulting in the highest extraction yield (33.2%) as well as the highest content of phenolic (13.36 mg GAE/g DW), flavonoid (1.92 mg QE/g DW), alkaloid (1.40 mg AE/g DW), and terpenoids (1.25%, w/w).
What ethanol is used for?
Ethanol is used in the manufacture of drugs, plastics, lacquers, polishes, plasticizers, and cosmetics. Ethanol is used in medicine as a topical antiinfective, and as an antidote for ethylene glycol or methanol overdose.
Can you drink ethanol?
Ethanol, or ethyl alcohol, is the only type of alcohol that you can drink without seriously harming yourself, and then only if it hasn’t been denatured or doesn’t contain toxic impurities. Ethanol is sometimes called grain alcohol because it is the main type of alcohol produced by grain fermentation.
Alcohol Ethanol Based Extraction of Cannabis
Images related to the topicAlcohol Ethanol Based Extraction of Cannabis

What is ethanol made from?
Ethanol is a domestically produced alternative fuel most commonly made from corn. It is also made from cellulosic feedstocks, such as crop residues and wood—though this is not as common. U.S. ethanol plants are concentrated in the Midwest because of the proximity to corn production.
Why is ethanol a bad solvent for extraction?
Methanol and ethanol are not useful extraction solvents because they are miscible with water and will not form a separate layer. Chloroform and methylene chloride are denser than water, while most other organic solvents are not as dense as water.
Why is ethanol used as solvent and not water?
The ethyl (C2H5) group in ethanol is non-polar. Ethanol therefore attracts non-polar molecules. Thus, ethanol can dissolve both polar and non-polar substances. In industrial and consumer products, ethanol is the second most important solvent after water.
Why is ethyl alcohol used as a solvent for extraction and not water?
Ethanol, also referred to as Ethyl alcohol (EtOH), is a common solvent which closely reproduces chemical ratios when used during extraction processes. Alcohol allows extraction of both water soluble and oil soluble components.
Why is 70% ethanol used?
70% denatured alcohol upholds key requirements for use as a bactericidal in clean rooms or medical facilities, but also for general purposes. 70% denatured ethanol mixed with 30% water solutions produce less vapour and odour, therefore reducing risks of toxic fumes or combustion.
Can you drink 100% ethanol?
While ethanol is consumed when drinking alcoholic beverages, consuming ethanol alone can cause coma and death. Ethanol may also be a carcinogenic; studies are still being done to determine this. However, ethanol is a toxic chemical and should be treated and handled as such, whether at work or in the home.
Is vodka an ethanol?
Distilled spirits (whisky, gin, vodka) usually contain 40–50% ethanol; wines contain 10–12% ethanol and beer ranges from 2–6% ethanol, while standard lager contains about 4% ethanol.
Why is methanol a better solvent than ethanol?
Methanol is the best solvent system to extract phytochemicals. Because methanol has high extractability in compare with Ethanol. methanol and its polarity work on vast number of phytochemicals including Polar and non polar compounds. the extractability we can assure atleast 50% on both side of the polarity.
Separating Components of a Mixture by Extraction
Images related to the topicSeparating Components of a Mixture by Extraction
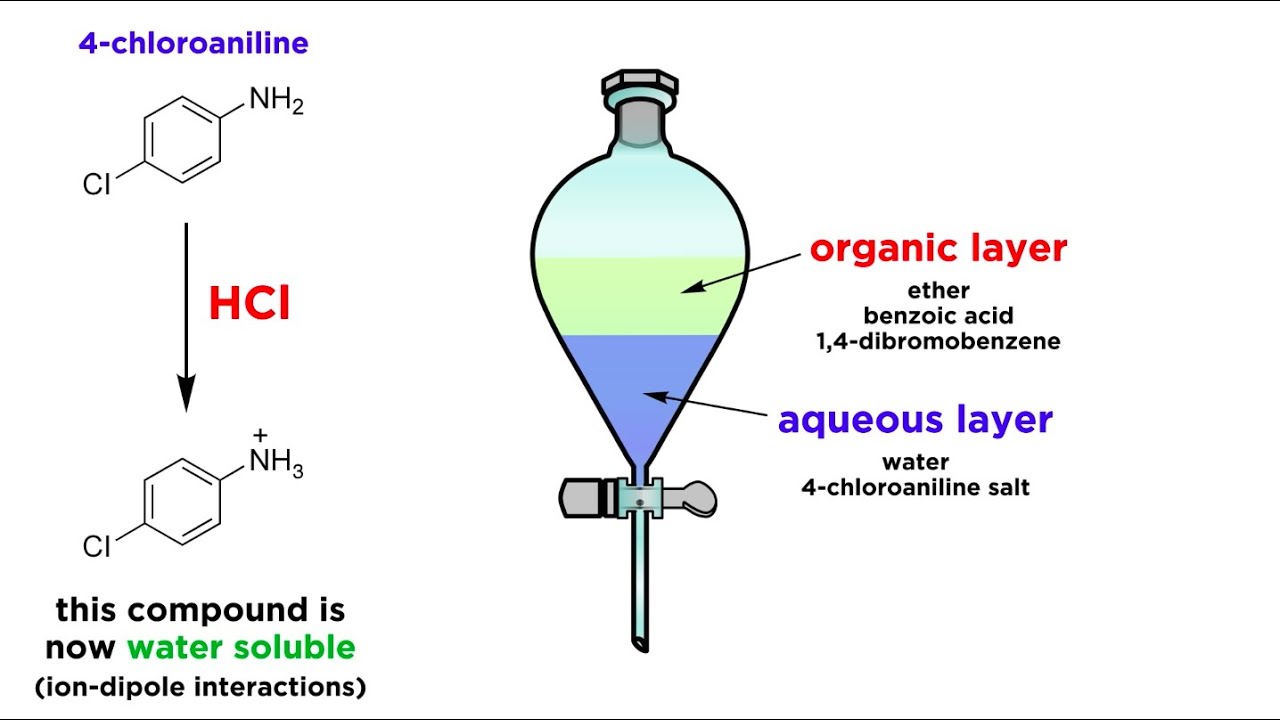
Which solvent is used in solvent extraction?
Organic solvents with low polarity such as hexanes, toluene, dichloromethane and diethyl ether are usually chosen as the organic extracting solvent.
Why is methanol used in extraction?
The polarity of the solvent used will determine the kind of compounds that can be extracted. Most at times, methanol is a polar solvent used to extract bioactive compounds from extracts and has very good yields when compared to other solvents (methanol has better yields than ethanol).
Related searches to What is extraction solvent ethanol?
- ethanol extraction of plants procedure
- which solvent is best for extraction
- ethanol extraction soak time
- what is extraction solvent ethanol
- which solvent is used in solvent extraction
- why 95 ethanol is used in plant extraction
- ethanol extraction method pdf
- is ethanol extraction safe
- why is ethanol used as solvent for extraction
- how is ethanol extracted
- diy ethanol extraction
- denatured ethanol for extraction
- ethanolic extraction method
Information related to the topic What is extraction solvent ethanol?
Here are the search results of the thread What is extraction solvent ethanol? from Bing. You can read more if you want.
You have just come across an article on the topic What is extraction solvent ethanol?. If you found this article useful, please share it. Thank you very much.