Are you looking for an answer to the topic “What is fluorine electron affinity?“? We answer all your questions at the website Ecurrencythailand.com in category: +15 Marketing Blog Post Ideas And Topics For You. You will find the answer right below.
Electron Affinity of Fluorine is 328 kJ/mol.Fluorine has highest electron affinity in the periodic table.Electron affinity is the energy released when 1 mole of gaseous atoms each acquire an electron to form 1 mole of gaseous 1- ions. Fluorine has a negative value as energy is released when the electron is gained. This is because there is an attraction between the protons in the nucleus and the added electrons.

Table of Contents
Is fluorine high in electron affinity?
Fluorine has highest electron affinity in the periodic table.
Why is electron affinity of fluorine negative?
Electron affinity is the energy released when 1 mole of gaseous atoms each acquire an electron to form 1 mole of gaseous 1- ions. Fluorine has a negative value as energy is released when the electron is gained. This is because there is an attraction between the protons in the nucleus and the added electrons.
Fluorine has lower electron affinity than chlorine because of
Images related to the topicFluorine has lower electron affinity than chlorine because of

Does fluorine have a low electron affinity?
Less energy is released. However, there are exceptions. Fluorine, which is higher up the group then chlorine, has a lower electron affinity.
Which has highest electron affinity?
Halogens has higher electron affinity and it is supposed to be for fluorine, but chlorine has higher electron affinity than fluorine due to fluorine’s smaller size. Hence, among given options chlorine has highest electron affinity.
How do you find the electron affinity of fluorine?
- Electron Affinity of Fluorine is 328 kJ/mol.
- First Ionization Energy of Fluorine is 17.4228 eV. …
- X + e– → X– + energy Affinity = – ∆H. …
- Affinities of Nonmetals vs.
Why is electron affinity of fluorine less than chlorine?
Electron affinity of fluorine is less than that of chlorine. This is due to the reason explained below: Fluorine has seven electrons in 2p-subshell whereas chlorine has seven electrons in its 3p-subshell. 3p-subshell is relatively larger than 2p-subshell.
Why does fluorine have a higher electron affinity than oxygen?
Increase in nuclear charge increases the effective force on the valence electron and hence the incoming electron can be held strongly and hence oxygen will have less electron affinity or fluorine will have greater electron affinity.
See some more details on the topic What is fluorine electron affinity? here:
Electron Affinity – Chemistry LibreTexts
A fluorine atom has an electronic structure of 1s22s22px22py22pz1. It has 9 protons in the nucleus.The incoming electron …
Electron Affinity of Fluorine
fluorine to be 83.2 kcal/mole with an uncertainty of. ±O. 3 kcal/mole. CALCULATION OF THE ELECTRON AFFINITY. FROM ATOMIC ENERGY LEVELS.
electron affinity – Chemguide
Fluorine breaks that pattern, and will have to be accounted for separately. The electron affinity is a measure of the attraction between the incoming …
What Is Electron Affinity? | Trends & Chart | ChemTalk
Fluorine presents another caveat regarding the group electron affinity trend. Although first electron affinities generally decrease as we …
What do you mean by electron affinity?
electron affinity, in chemistry, the amount of energy liberated when an electron is added to a neutral atom to form a negatively charged ion. The electron affinities of atoms are difficult to measure, hence values are available for only a few chemical elements, chiefly the halogens.
What is the electronegativity of F?
How do you find electron affinity?
The amount of energy released when a neutral atom in its gaseous state accepts an electron and gets converted into a negatively charged ion is known as electron affinity. X ( g ) + e − → X − ( g ) + E . A . Therefore, the electron affinity of chlorine is – 349 KJ/mol.
Electron affinity: period trend | Atomic structure and properties | AP Chemistry | Khan Academy
Images related to the topicElectron affinity: period trend | Atomic structure and properties | AP Chemistry | Khan Academy
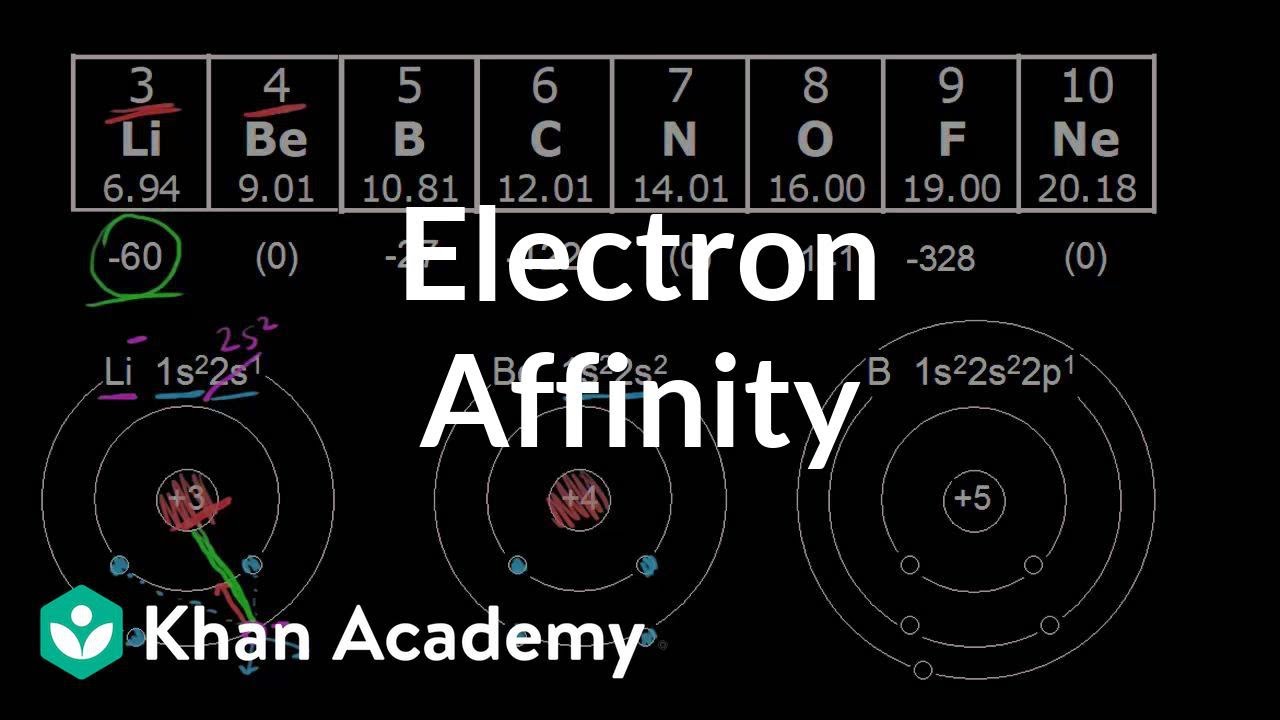
What is electron affinity trend?
Electron affinity generally increases across a period in the periodic table and sometimes decreases down a group. These trends are not necessarily universal. The chemical rationale for changes in electron affinity across the periodic table is the increased effective nuclear charge across a period and up a group.
Which has more electron affinity fluorine or chlorine?
Electronegativity of fluorine is greater than that of chlorine but electron affinity of chlorine is greater than that of fluorine.
What has the lowest electron affinity?
The correct answer is Argon. Argon has all filled orbitals as well as a filled valence shell. As a result, it doesn’t want to lose or gain any electrons. Hence, argon has the lowest electron affinity.
Which has higher electron affinity fluorine or neon?
Thus, noble gases have the least electron affinity in a period. Hence, we can conclude that in a period, fluorine (halogen) has higher electron affinity than neon (noble gas).
What is electron affinity and electronegativity?
Electronegativity is defined as a chemical property which decides the propensity of an atom to attract an electron. In the year 1932, Linus Pauling proposed the concept of electronegativity. Electron affinity is defined as the amount of energy liberated when a molecule or neutral atom acquires an electron from outside.
What is chlorine electron affinity?
Electron Affinity of Chlorine is 349 kJ/mol.
What is the electron gain enthalpy of fluorine?
| Element | Electron gain enthalpy (KJ/mol) |
|---|---|
| Fluorine | -328 |
| Chlorine | -349 |
| Bromine | -325 |
| Iodine | -295 |
Why electron affinity decreases down the group?
When moving down a group, the electron affinity generally decreases. This is because as you go down the period table, new valence shells are added increasing the atomic radius. The new orbital is further away from the nucleus, meaning the attraction between the positively charged nucleus and the new electron decrease.
Electron Affinity Trend, Basic Introduction, Chemistry
Images related to the topicElectron Affinity Trend, Basic Introduction, Chemistry
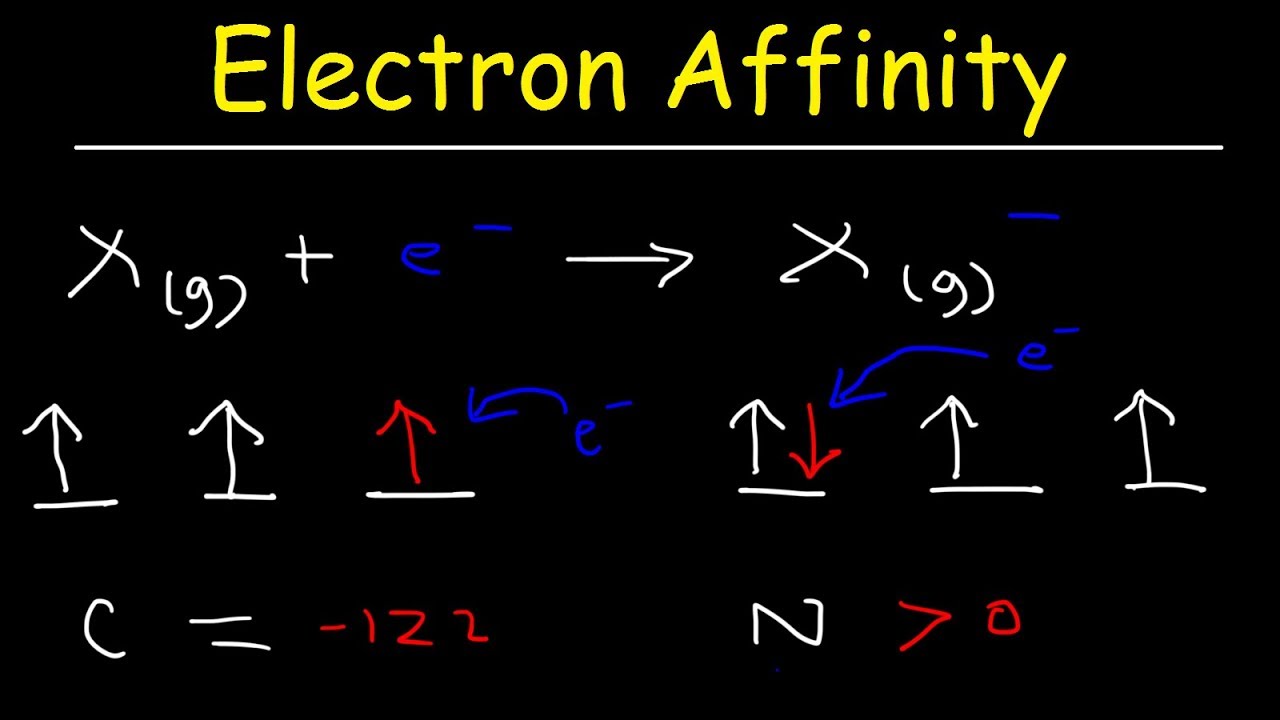
Why do halogens have high electron affinity?
The atomic size of halogens is very small. The smaller the atomic size , the greater the electron affinity , because the effective attractive force between the nucleus and the valence electrons is greater in smaller atoms , and so the electrons are held firmly.
On what factors does electron affinity depends?
The three factors affecting the electron affinity of a molecule are Nuclear Charge, Atomic Size, and Electronic Configuration. Nuclear Charge: The greater the nuclear charge, the greater will be the attraction of the incoming electron. This will result in a larger value of electron affinity.
Related searches to What is fluorine electron affinity?
- does fluorine have the highest electron affinity
- what is the reaction that corresponds to the electron affinity of fluorine
- chlorine electron affinity
- electron affinity trend down a group
- why does fluorine have a high electron affinity
- electron affinity table
- electron affinity of nitrogen
- lowest electron affinity
- what is the electron affinity of fluorine
- does fluorine have a high electron affinity
- electron affinity value of fluorine
- electron affinity trend
- what is the reaction that corresponds to the electron affinity of fluorine f
- electron affinity of oxygen
- electron affinity of sodium
Information related to the topic What is fluorine electron affinity?
Here are the search results of the thread What is fluorine electron affinity? from Bing. You can read more if you want.
You have just come across an article on the topic What is fluorine electron affinity?. If you found this article useful, please share it. Thank you very much.
