Are you looking for an answer to the topic “What is the amount in moles of 11.8 g Ar?“? We answer all your questions at the website Ecurrencythailand.com in category: +15 Marketing Blog Post Ideas And Topics For You. You will find the answer right below.
So for instance, if we have 11.8 g of argon, We can say that every one mole of Oregon has a mass of 39.95 g. Again coming right off the periodic table grams, cancel out your left with molds. We have three significant figures here. So we’re gonna say point 295 moles.Using the older standard, as indicated in the explanation, 11.2 L of Ar atoms contain 0.500 mol .Solution : Number of moles of sodium atoms `=(“mass of sodium “)/(“Gram Atomic Weight”) = (11.5)/(23) = 0.5` <br> i.e., 0.5 moles of Na atoms are there in the given 11.5 g sample.

How many moles are in 11.2 g Ar?
Using the older standard, as indicated in the explanation, 11.2 L of Ar atoms contain 0.500 mol .
How many moles are in 11.5 g?
Solution : Number of moles of sodium atoms `=(“mass of sodium “)/(“Gram Atomic Weight”) = (11.5)/(23) = 0.5` <br> i.e., 0.5 moles of Na atoms are there in the given 11.5 g sample.
Avogadro’s Number, The Mole, Grams, Atoms, Molar Mass Calculations – Introduction
Images related to the topicAvogadro’s Number, The Mole, Grams, Atoms, Molar Mass Calculations – Introduction

What is the moles of Ar?
The symbol for argon is Ar, and its atomic number is 18. More importantly for the purposes of making our converter, the atomic mass of argon is 39.948. That means that one mole of argon weighs 39.948 grams (39.948 g/mol).
How many moles are in 22.0 grams of argon?
number of moles of Ar = 22 g / 40 g/mol = 0.55 mol .
How many moles are in 11.2 L of a gas mol?
It’s a fact that one mole of ANY gas at STP occupies 22.4 L of space, so 11.2 L = 0.5 moles.
How many moles does 11.2 L of hydrogen gas contain at STP?
Answer: Hence, the number of moles, molecules and atoms present in 11.2 liters of H2 at STP are 0.5 , 3.011 x 1023 and 6.022 x 1023 respectively.
How many moles are there in 11.5 g of C2H5OH?
The answer is 46.06844. We assume you are converting between grams C2H5OH and mole. You can view more details on each measurement unit: molecular weight of C2H5OH or mol The SI base unit for amount of substance is the mole.
See some more details on the topic What is the amount in moles of 11.8 g Ar? here:
How many moles are in 11.8g Ar? – Answers
The Atomic Mass of Ar is 39.9Amount of Ar = mass of pure sample/molar mass = 11.8/39.9 = 0.296mol There are 0.296 moles of argon in a 11.8g …
Convert grams Argon to moles – Conversion of Measurement …
The answer is 39.948. We assume you are converting between grams Argon and mole. You can view more details on each measurement unit: molecular weight of Argon …
What is the amount, in moles, of each elemental … – Study.com
Here molecular weight of. Ar=39.95 gm. Zn=63.38 gm. Ti=47.867 gm. Li= 6.941 gm. is given so from above given data we can calculate. a) moles in 11.8g…
What is the number of atoms in 11.5 g of sodium?
Answer. 3.011*10^23 atoms. So there are 3.011*10^23 atoms present in 11.5g of sodium sample.
How do I calculate moles?
- Measure the weight of your substance.
- Use a periodic table to find its atomic or molecular mass.
- Divide the weight by the atomic or molecular mass.
- Check your results with Omni Calculator.
How many grams are in 4.35 moles of Ar?
Argon has a mass of 39.95 g⋅mol−1 . If there are 4.35 mol, then the mass is simply 4.35 mol×39.95⋅g⋅mol−1 . Note that as an inert gas, we quite properly assume that argon is atomic.
How To Convert Grams To Moles – VERY EASY!
Images related to the topicHow To Convert Grams To Moles – VERY EASY!
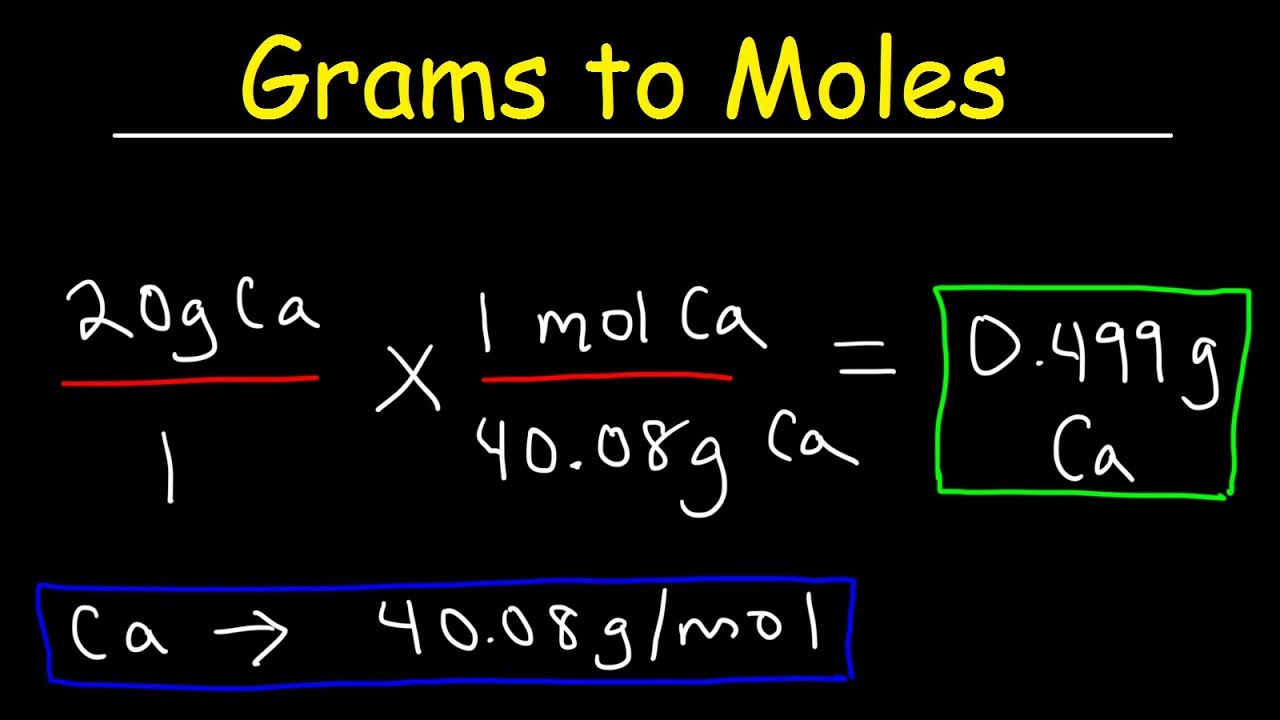
How do you calculate number of moles?
- The formula for the number of moles formula is expressed as.
- Given.
- Number of moles formula is.
- Number of moles = Mass of substance / Mass of one mole.
- Number of moles = 95 / 86.94.
How many moles are in 11.9 grams of chromium?
Furthermore, the atomic mass of chromium is 51.996. That means that one mole of chromium weighs 51.996 grams (51.996 g/mol). Based on that information, to convert 11.9 moles of chromium to grams, we multiply 11.9 moles of chromium by 51.996.
How many moles are in 50.5 g of argon?
The symbol for argon is Ar, and its atomic number is 18. More importantly for the purposes of making our converter, the atomic mass of argon is 39.948. That means that one mole of argon weighs 39.948 grams (39.948 g/mol).
How many moles are in 452 grams of argon?
The number of moles in 452 grams of Argon is 13.31 moles.
How many moles are in 11.2 L of oxygen?
Answer. 11.2/22.4=0.5 moles.
How many moles of propane gas would be present in 11 grams of the gas at standard conditions?
The volume occupied by one mole of a gas at standard correlation. The mass of two moles of propane gas, C3H8. The number of moles of propane gas, C3H8, are contained in 11 grams of the gas at standard conditions.
How many grams of oxygen are in 11.2 L o2 gas at STP?
0.5 moles x 32 g/mole = 16 g. You were correct.
How many moles of n2 gas occupy a volume of 11.2 liters at STP?
0.5 moles⋅22.4 L/mol=11.2 L , and so on.
Stoichiometry Mole to Mole Conversions – Molar Ratio Practice Problems
Images related to the topicStoichiometry Mole to Mole Conversions – Molar Ratio Practice Problems
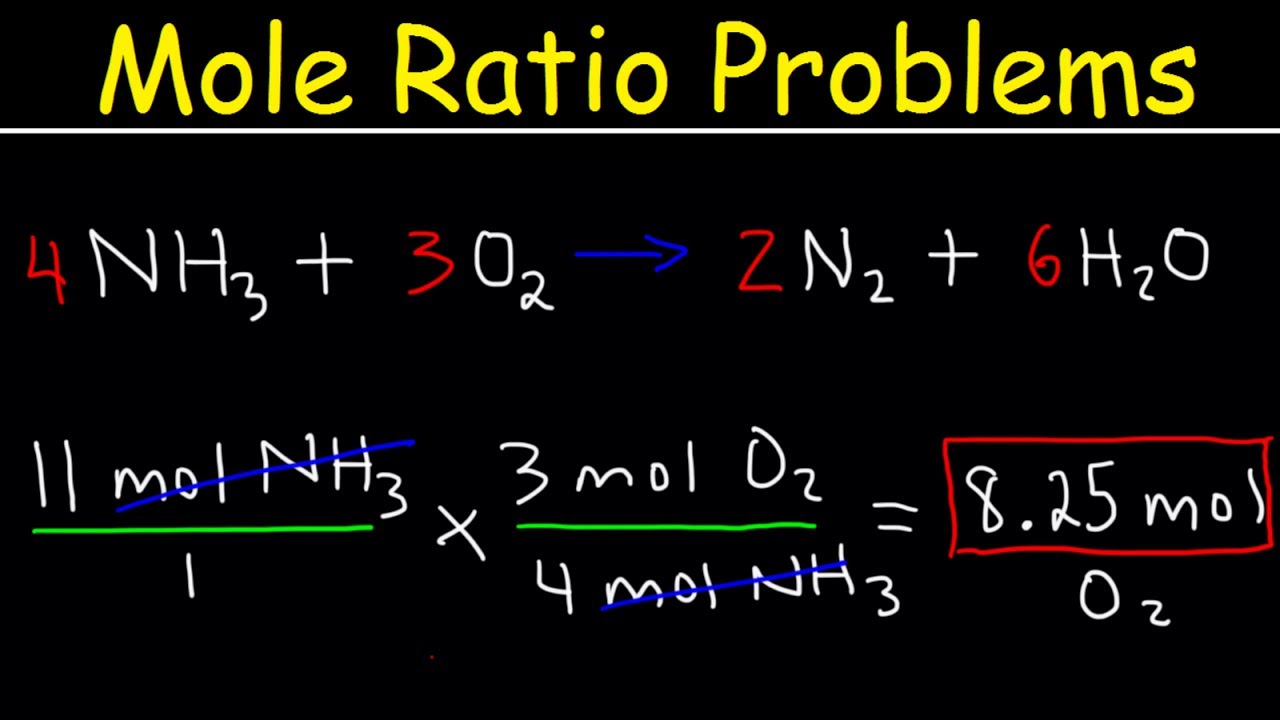
How many moles of H2 are present?
H2 – There are 2 moles of hydrogen atoms (1 mole of elemental hydrogen). Total: 2 moles of atoms.
What is the volume occupied by 0.25 mol of a gas at STP?
1 Answer. 0.25 mole of NO2 will occupy 5.6 litres of volume.
Related searches to What is the amount in moles of 11.8 g Ar?
- How to Calculate number of atoms
- 0.211 g li to moles
- Gram to mole
- what is the amount in moles of 11 8 g ar to moles
- how to calculate number of atoms
- mol to liter
- what is the amount in moles of 11 8 g ar to mol
- 0 211 g li to moles
- Mol to liter
- Mol to gram
- 26 1 g ta in moles
- gram to mole
- 26.1 g ta in moles
- what is the mass in grams of each elemental sample
- how many silver atoms are there in 3 78 g of silver
- mol to gram
Information related to the topic What is the amount in moles of 11.8 g Ar?
Here are the search results of the thread What is the amount in moles of 11.8 g Ar? from Bing. You can read more if you want.
You have just come across an article on the topic What is the amount in moles of 11.8 g Ar?. If you found this article useful, please share it. Thank you very much.