Are you looking for an answer to the topic “What is the angle of a water molecule?“? We answer all your questions at the website Ecurrencythailand.com in category: +15 Marketing Blog Post Ideas And Topics For You. You will find the answer right below.
The actual bond angle in the water molecule is 104.5°.There is electron-electron repulsion between these two regions of electron density. This repulsion forces the hydrogen atoms away from the oxygen electron pairs, giving deviation from 180 degrees. Hope this helped!The molecule adopts a bent structure because of the two lone pairs of electrons on the oxygen atom. The H−O−H bond angle is about 105o, slightly smaller than the ideal 109.5o of an sp3 hybridized atomic orbital.

Why is the bond angle of H2O not 180?
There is electron-electron repulsion between these two regions of electron density. This repulsion forces the hydrogen atoms away from the oxygen electron pairs, giving deviation from 180 degrees. Hope this helped!
Why is water molecule at an angle?
The molecule adopts a bent structure because of the two lone pairs of electrons on the oxygen atom. The H−O−H bond angle is about 105o, slightly smaller than the ideal 109.5o of an sp3 hybridized atomic orbital.
Water Molecular Geometry and Bond Angles
Images related to the topicWater Molecular Geometry and Bond Angles

Why is water molecule 104.5 degrees?
A water molecule consists of two hydrogen atoms and one oxygen atom. The three atoms make an angle; the H-O-H angle is approximately 104.5 degrees. The center of each hydrogen atom is approximately 0.0957 nm from the center of the oxygen atom.
How do you find the bond angle of H2O?
The bond angle in H2O is 104.5^o .
Is h2o bent?
Polarity of water molecules
A water molecule consists of two hydrogen atoms bonded to an oxygen atom, and its overall structure is bent.
What is a 104.5 bond angle?
An oxygen atom has 6 electrons in which two of them are bonded with a hydrogen atom leaving two lone pairs of electrons. Due to the presence of these lone pairs of electrons in H2O, the bond angle is 104.50. This can be explained with the help of valence shell electron pair repulsion theory (VSEPR).
What has a bond angle of 107?
The bond angle in a molecule of ammonia (NH3) is 107 degrees so why, when part of a transition metal complex is the bond angle 109.5 degrees.
See some more details on the topic What is the angle of a water molecule? here:
FAQ: Water molecular structure – IAPWS
A water molecule consists of two hydrogen atoms and one oxygen atom. The three atoms make an angle; the H-O-H angle is approximately 104.5 …
Water and its structure – Chem1
This would ordinarly result in a tetrahedral geometry in which the angle between electron pairs (and therefore the H-O-H bond …
Why is the bond angle of water molecule 109 and not 180?
The bond angle of water is 104.45 (degrees). A very simplified answer not involving molecular orbitals: On the left is he carbon dioxide molecule.
15.1: Structure of Water – Chemistry LibreTexts
The molecule adopts a bent structure because of the two lone pairs of electrons on the oxygen atom. The H−O−H bond angle is about 105o, …
What shape has a bond angle of 107?
Ammonia molecule. Recall that the bond angle in the tetrahedral CH 4 molecule is 109.5°. Again, the replacement of one of the bonded electron pairs with a lone pair compresses the angle slightly. The H-N-H angle is approximately 107°.
Bond Angles for H2O (Ideal and Actual)
Images related to the topicBond Angles for H2O (Ideal and Actual)

What is the bond angle and geometry of water?
In agreement with our analysis using the Lewis formalism, water’s shape is angular, or bent, with an H-O-H bond angle of 104.5°. This is consistent with a roughly tetrahedral orientation of four electron domains about the central oxygen, two bonding pairs and two non-bonding “lone pairs”.
What is bond angle of H-O-H in H2O molecule?
The H-O-H angle in a water molecule is about 105∘ due to two lone pairs of the electron.
Why is a water molecule V-shaped?
The hydrogen and oxygen atoms join to produce a V-shaped molecule, with hydrogen atoms on one end and oxygen on the other. This configuration produces a highly polar molecule, having a positive charge on the hydrogen end and a negative on the oxygen end.
Is H2O linear in shape?
H2O molecule is V-shaped and not linear.
Why is water molecule bent shaped?
Explanation: In the Lewis structure for H2O , the central oxygen atom is bonded to two hydrogen bonds. The bonds between oxygen and hydrogen are single covalent bonds. The central oxygen atom has two lone pairs of electrons, which is why the water molecule has a bent shape.
What is hybridization of H2O?
If we look at the general rule of hybridization it states that only the central atom undergoes the hybridization process. During the formation of a water molecule, we focus on the oxygen atom. In hybridization of H2O, the oxygen atom is sp3 hybridized.
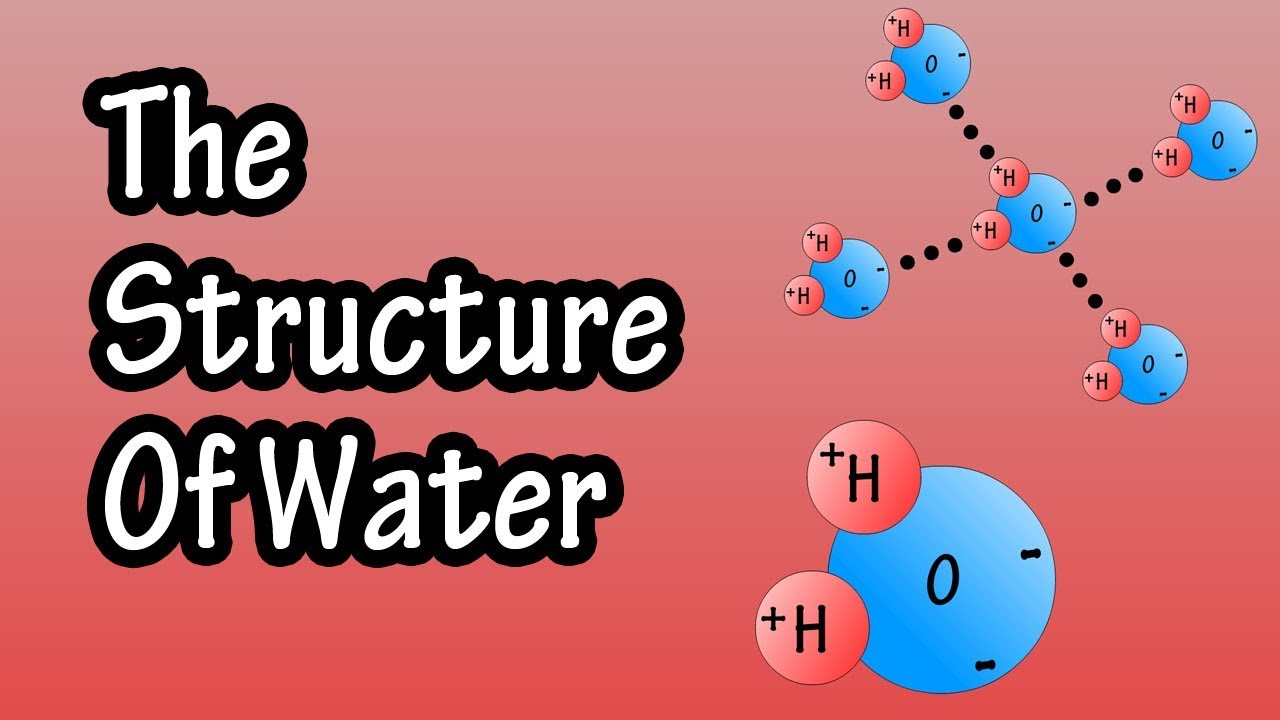
Structure Of Water Molecule – Chemistry Of Water – Properties Of Water – Composition Of Water
Images related to the topicStructure Of Water Molecule – Chemistry Of Water – Properties Of Water – Composition Of Water
What type of bond is h20?
Water (H2O), like hydrogen fluoride (HF), is a polar covalent molecule.
What is the bond angle of ice?
The angle between bonds in the crystal lattice is very close to the tetrahedral angle of 109.5°, which is also quite close to the angle between hydrogen atoms in the water molecule (in the gas phase), which is 105°.
Related searches to What is the angle of a water molecule?
- what is the geometric structure of the water molecule
- what is the bond angle of a water molecule
- what is contact angle of water
- structure of water pdf
- water bond angle and shape
- shape of water molecule
- how are atoms in a water molecule bonded together
- tetrahedral structure of water
- how is a water molecule held together
- what is the angle between the bonds of a water molecule
- what is the angle of water molecule
- what is the angle of water
- what is the geometry of water molecule
- what is bond angle in water
- 5 water molecules
- the water molecule is bent with a bond angle of what (list the value here)
- what is the structure of water molecule
Information related to the topic What is the angle of a water molecule?
Here are the search results of the thread What is the angle of a water molecule? from Bing. You can read more if you want.
You have just come across an article on the topic What is the angle of a water molecule?. If you found this article useful, please share it. Thank you very much.