Are you looking for an answer to the topic “What is the atomic mass of boron 11?“? We answer all your questions at the website Ecurrencythailand.com in category: +15 Marketing Blog Post Ideas And Topics For You. You will find the answer right below.
boron-11 atom (CHEBI:52451) The stable isotope of boron with relative atomic mass 11.009306, 80.1 atom percent natural abundance and nuclear spin 3/2.What is the atomic mass of boron? The mass of an average boron atom, and thus boron’s atomic mass, is 10.8amu.Boron-11 | B – PubChem.
| Atomic Number | Symbol | Atomic Weight (amu, g/mol) |
|---|---|---|
| 11 | Na | 22.98977 |
| 12 | Mg | 24.305 |
| 13 | Al | 26.98154 |
| 14 | Si | 28.0855 |
| PubChem CID | 58665377 |
|---|---|
| Structure | Find Similar Structures |
| Molecular Formula | B |
| Molecular Weight | 12.01435 |
| Dates | Modify 2022-05-21 Create 2012-08-19 |

What is the atomic mass of element 11?
| Atomic Number | Symbol | Atomic Weight (amu, g/mol) |
|---|---|---|
| 11 | Na | 22.98977 |
| 12 | Mg | 24.305 |
| 13 | Al | 26.98154 |
| 14 | Si | 28.0855 |
Is the atomic mass of boron-10 or 11?
What is the atomic mass of boron? The mass of an average boron atom, and thus boron’s atomic mass, is 10.8amu.
boron has two isotopes,B-11. The average atomic mass of boron is found to be 10.80u.
Images related to the topicboron has two isotopes,B-11. The average atomic mass of boron is found to be 10.80u.

How do you write boron-11?
Boron-11 | B – PubChem.
What is the atomic mass of boron 12?
| PubChem CID | 58665377 |
|---|---|
| Structure | Find Similar Structures |
| Molecular Formula | B |
| Molecular Weight | 12.01435 |
| Dates | Modify 2022-05-21 Create 2012-08-19 |
How do u calculate atomic mass?
Together, the number of protons and the number of neutrons determine an element’s mass number: mass number = protons + neutrons. If you want to calculate how many neutrons an atom has, you can simply subtract the number of protons, or atomic number, from the mass number.
What is atomic mass of element?
atomic mass, the quantity of matter contained in an atom of an element. It is expressed as a multiple of one-twelfth the mass of the carbon-12 atom, 1.992646547 × 10−23 gram, which is assigned an atomic mass of 12 units. In this scale, 1 atomic mass unit (amu) corresponds to 1.660539040 × 10−24 gram.
How many neutrons are in boron-11?
So boron-11 has five protons the same as boron-10. Then the mass number is total protons plus neutrons. For boron-11 this total is 11, and five of the particles are protons, thus 11−5=6 neutrons.
See some more details on the topic What is the atomic mass of boron 11? here:
4.9: Atomic Mass: The Average Mass of an Element’s Atoms
The mass of an average boron atom, and thus boron’s atomic mass, is 10.8amu. Example 4.9.2: Neon Isotopes. Neon has three naturally occurring …
Atomic Weight of Boron
In 1961, the Commission recommended Ar(B) = 10.811(3) based on calibrated mass-spectrometric measurements on brines and minerals from Searles Lake.
Boron-11 Isotope | AMERICAN ELEMENTS ®
Boron (atomic symbol: B, atomic number: 5) is a Block P, Group 13, Period 2 element with an atomic weight of 10.81. The number of electrons in each of boron’s …
Atomic Number – Atomic Mass – Density of Boron – What is …
Atomic mass of Boron is 10.811 u. … Note that each element may contain more isotopes. Therefore this resulting atomic mass is calculated from …
What is the atomic mass of boron-10?
| ChEBI Name | boron-10 atom |
|---|---|
| ChEBI ID | CHEBI:77014 |
| Definition | A stable isotope of boron with relative atomic mass 10.0129370, 19.9 atom percent natural abundance and nuclear spin 3+. |
| Stars | This entity has been manually annotated by the ChEBI Team. |
| Submitter | Philippe Rocca-Serra |
How many electrons does boron-11 have?
That means it contains five protons and five electrons.
What is isotope b11?
Boron 11 Metal (Boron-11) is a stable (non-radioactive) isotope of Boron. It is both naturally occurring and a produced by fission. Boron 11 Metal is one of over 250 stable Metallic isotopes produced by American Elements for biological and biomedical labeling, as target materials and other applications.
Has a mass number of 11 and an atomic number 5?
This means that any atom that has 5 protons in its nucleus will be a boron atom. So, the mass number of this boron isotope is equal to 11 , and the atomic number to 5 .
How to Calculate the Average Atomic Mass of Boron
Images related to the topicHow to Calculate the Average Atomic Mass of Boron
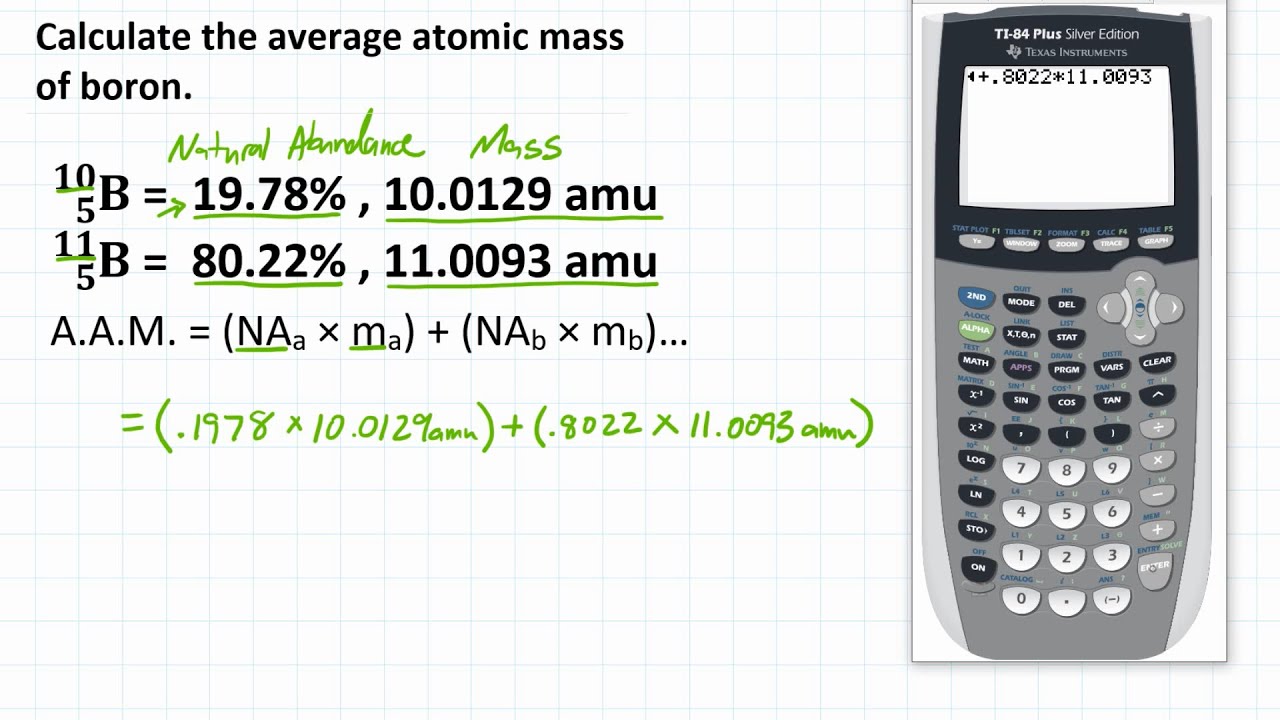
Why is boron-11 more abundant?
The atomic mass of boron is 10.81 u. And 10.81 u is a lot closer to 11u than it is to 10u, so there must be more of boron-11. Where u is the unit for atomic mass and x is the proportion of boron-10 out of the total boron abundance which is 100%. And thus the abundance of boron-11 is roughly 81%.
What is the mass number of boron 14?
| Nuclide | Z | Isotopic mass (Da) |
|---|---|---|
| Excitation energy | ||
| 12 B | 5 | 12.0143526(14) |
| 13 B | 5 | 13.0177800(11) |
| 14 B | 5 | 14.025404(23) |
How do you find the atomic mass of boron?
The atomic mass of boron is 10.81. Note that this is the value listed in the periodic table for the atomic mass of boron. Although the atomic number of boron is 10, its atomic mass is nearer to 11 than to 10, reflecting the fact that the heavier isotope is more abundant than the lighter isotope.
How do you calculate atomic number?
The atomic number of an atom is equal to the number of protons in the nucleus of an atom or the number of electrons in an electrically neutral atom. For example, in a sodium atom, there are 11 electrons and 11 protons. Thus the atomic number of Na atom = number of electrons = number of protons = 11.
Is mass number and atomic mass same?
The mass number is the sum of the number of protons and neutrons in an atom. It is a whole number. The atomic mass is the average number of protons and neutrons for all natural isotopes of an element. It is a decimal number.
What is the atomic number?
atomic number, the number of a chemical element in the periodic system, whereby the elements are arranged in order of increasing number of protons in the nucleus. Accordingly, the number of protons, which is always equal to the number of electrons in the neutral atom, is also the atomic number.
What is the atomic mass of 1 to 20 elements?
…
Atomic Number of Elements from 1 to 30.
| Atomic Number | Element | Atomic Mass |
|---|---|---|
| 18 | Argon | 39.948 |
| 19 | Potassium | 39.098 |
| 20 | Calcium | 40.078 |
| 21 | Scandium | 44.956 |
What is the atomic mass of 1 to 30 elements?
| ATOMIC NUMBER | ELEMENT | ATOMIC MASS |
|---|---|---|
| 1 | Hydrogen | 1.008 |
| 2 | Helium | 4.0026 |
| 3 | Lithium | 6.94 |
| 4 | Beryllium | 9.0122 |
What is the difference between boron-10 and Boron-11?
Natural occurring boron has two stable isotopes: boron-10 and boron-11. The natural abundance of boron 10 is about 19.8%, and the natural abundance of boron 11 is about 80.2%. The thermal neutron cross section of boron 10 is 3837 barn, and the thermal neutron cross section of boron 11 is less than 0.1 barn[1].
Boron has two isotopes, boron-10 and boron-11. Based on the average atomic mass, which isotope is
Images related to the topicBoron has two isotopes, boron-10 and boron-11. Based on the average atomic mass, which isotope is

What is the atomic number of boron?
What is the mass of a mole of boron atoms?
Furthermore, the atomic mass of boron is 10.81. That means that one mole of boron weighs 10.81 grams (10.81 g/mol).
Related searches to What is the atomic mass of boron 11?
- boron 11 protons neutrons electrons
- how to find the atomic mass of boron
- boron number of protons
- what is the atomic mass of boron 11
- what is the average atomic mass of boron – 11
- boron 11 number of neutrons
- boron 11 number of protons
- atomic mass of boron 10
- boron 10 and boron 11
- what is the atomic number of boron
- boron 11 electrons
- what is the relative atomic mass of boron 10 and 11
- boron-11 number of protons
- if 19.9 of boron is boron-10 and 80.1 is boron-11 what is the average atomic mass
- boron-10 and boron-11
Information related to the topic What is the atomic mass of boron 11?
Here are the search results of the thread What is the atomic mass of boron 11? from Bing. You can read more if you want.
You have just come across an article on the topic What is the atomic mass of boron 11?. If you found this article useful, please share it. Thank you very much.