Are you looking for an answer to the topic “What is the atomic structure called?“? We answer all your questions at the website Ecurrencythailand.com in category: +15 Marketing Blog Post Ideas And Topics For You. You will find the answer right below.
Atomic structure refers to the structure of an atom comprising a nucleus (centre) in which the protons (positively charged) and neutrons (neutral) are present. The negatively charged particles called electrons revolve around the centre of the nucleus.An atom is a complex arrangement of negatively charged electrons arranged in defined shells about a positively charged nucleus. This nucleus contains most of the atom’s mass and is composed of protons and neutrons (except for common hydrogen which has only one proton). All atoms are roughly the same size.Atoms are made up of 3 basic components known as subatomic particles, consisting of protons (positively charged), neutrons (no charge), and electrons (negatively charged). These are the parts of the atom. The atomic structure of these building blocks is very interesting.
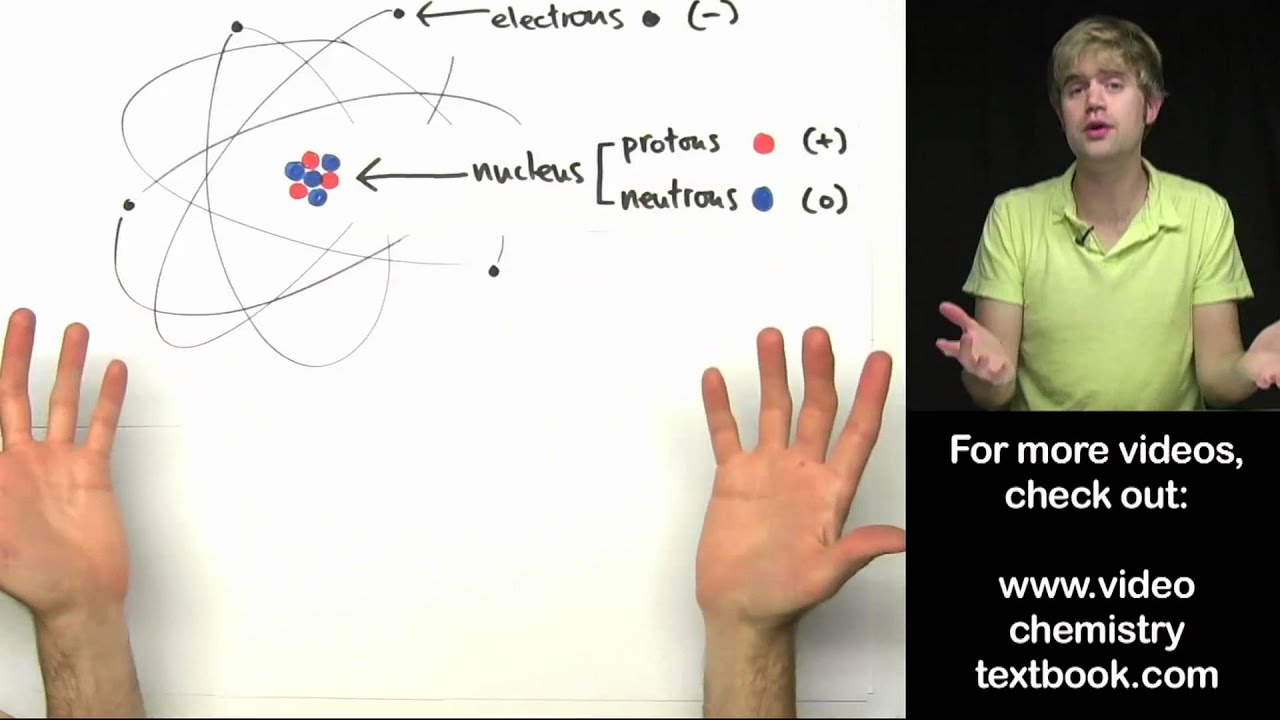
What is atom and its structure?
An atom is a complex arrangement of negatively charged electrons arranged in defined shells about a positively charged nucleus. This nucleus contains most of the atom’s mass and is composed of protons and neutrons (except for common hydrogen which has only one proton). All atoms are roughly the same size.
What are the 3 structure of an atom?
Atoms are made up of 3 basic components known as subatomic particles, consisting of protons (positively charged), neutrons (no charge), and electrons (negatively charged). These are the parts of the atom. The atomic structure of these building blocks is very interesting.
Basic Atomic Structure: A Look Inside the Atom
Images related to the topicBasic Atomic Structure: A Look Inside the Atom

What is atomic structure simple?
atomic structure. noun. the concept of an atom as a central positively charged nucleus consisting of protons and neutrons surrounded by a number of electrons. The number of electrons is equal to the number of protons: the whole entity is thus electrically neutral.
What is the structure of an atom quizlet?
atomic structure. The atom consists of three component parts: Protons, Neutrons, and Electrons. alpha particles. positively charged particles containing two protons and two neutrons, is identical to the nucleus of a helium atom and about 4 times the mass of a hydrogen atom. You just studied 21 terms!
What is structure of molecule?
Thus, from a structural point of view, a molecule consists of an aggregation of atoms held together by valence forces. Diatomic molecules contain two atoms that are chemically bonded.
Which statement best describes the structure of atoms?
Answer. The statement which best describes an atom is B. a group of protons and neutrons that are surrounded by electrons.
What is atomic structure and bonding?
From elementary chemistry it is known that the atomic structure of any element is made up of a positively charged nucleus surrounded by electrons revolving around it. An element’s atomic number indicates the number of positively charged protons in the nucleus.
See some more details on the topic What is the atomic structure called? here:
The Structure of the Atom | Boundless Chemistry
Overview of Atomic Structure. Atoms are made up of particles called protons, neutrons, and electrons, which are responsible for the mass and charge of atoms.
Atomic Structure – Atomic Archive
Atomic Structure. An atom is a complex arrangement of negatively charged electrons arranged in defined shells about a positively charged nucleus.
The Structure of an Atom | Protons, Neutrons, Electrons
Atoms are made up of 3 basic components known as subatomic particles, consisting of protons (positively charged), neutrons (no charge), …
Atomic Structure
Atoms are made of even smaller particles called protons, electrons, and neutrons. Protons and neutrons live in the nucleus of an atom and are almost identical …
Chemistry – Atomic Structure – EXPLAINED!
Images related to the topicChemistry – Atomic Structure – EXPLAINED!
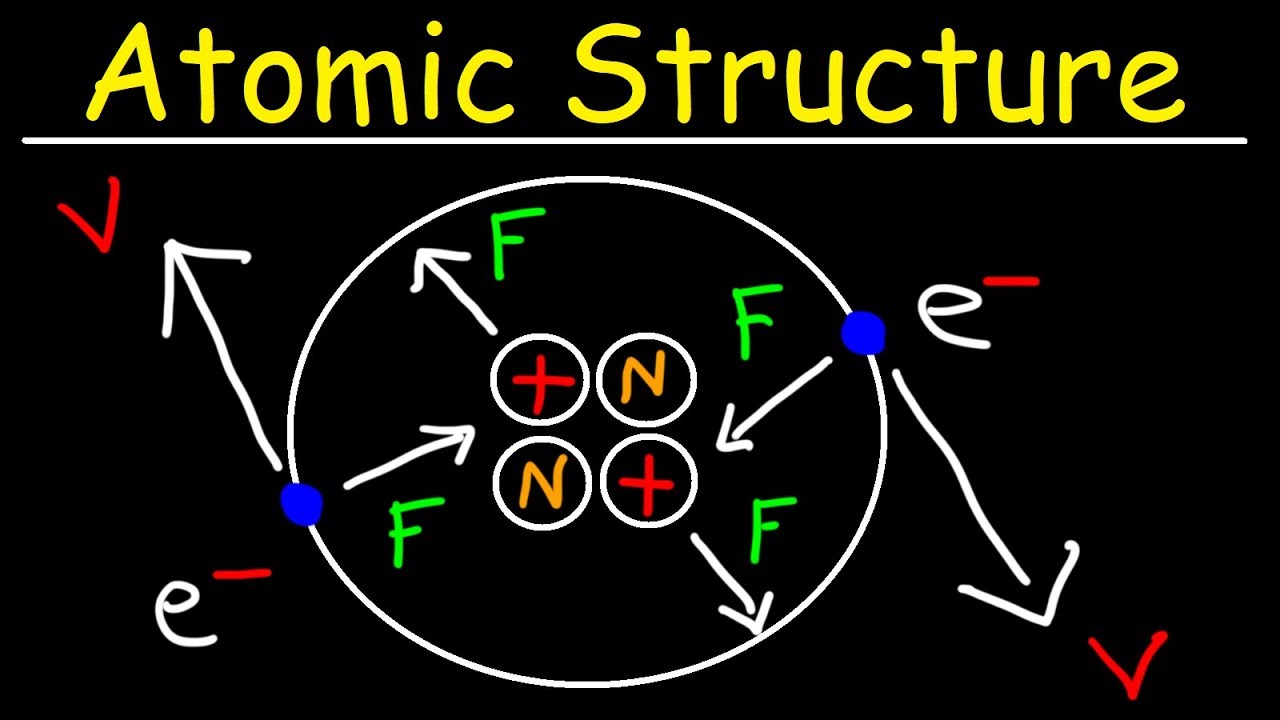
What is the center of an atom called?
The nucleus (or center) of an atom is made up of protons and neutrons. The number of protons in the nucleus, known as the “atomic number,” primarily determines where that atom fits on the Periodic Table.
What called atom?
An atom is the basic building block of chemistry. It is the smallest unit into which matter can be divided without the release of electrically charged particles. It also is the smallest unit of matter that has the characteristic properties of a chemical element.
Which structure is located in the center of the atom quizlet?
Positively charged subatomic particle located in the nucleus of the atom. Mass is 1 a.m.u. A subatomic particle located in the nucleus that has no charge.
What is the dense central core of an atom called?
The nucleus. The protons and neutrons are found tightly bound into a very small, positively charged, central core called the nucleus. In the average size atom, the nucleus takes up 100 000th of the diameter of the atom.
Which statement describes an atomic nucleus?
Which statement describes an atomic nucleus? An atomic nucleus contains most of the atom’s mass.
What are molecular structures called?
Molecular geometry, also known as the molecular structure, is the three-dimensional structure or arrangement of atoms in a molecule. Understanding the molecular structure of a compound can help determine the polarity, reactivity, phase of matter, color, magnetism, as well as the biological activity.
The 2,400-year search for the atom – Theresa Doud
Images related to the topicThe 2,400-year search for the atom – Theresa Doud

What is structure of an element?
The atomic structure of an element refers to the constitution of its nucleus and the arrangement of the electrons around it. Primarily, the atomic structure of matter is made up of protons, electrons and neutrons.
What is compound structure?
When atoms combine through chemical bonding, they form compounds—unique structures composed of two or more atoms. The basic composition of a compound can be indicated using a chemical formula.
Related searches to What is the atomic structure called?
- What is atom
- history of atom
- atomic structure
- atomic mass
- Structure of atom
- how to understand atomic structure
- how are atoms represented
- Atomic number
- what is molecule
- bohr atomic model
- Atomic structure
- Atomic mass
- what is the basic atomic structure
- structure of atom
- what is a in atomic structure
- what are the parts of the atomic structure
- Bohr atomic model
- how do you find the atomic structure
- what is atom
- atomic number
- what is the current model of atomic structure called
- how to describe atomic structure
- what is meant by atomic structure
Information related to the topic What is the atomic structure called?
Here are the search results of the thread What is the atomic structure called? from Bing. You can read more if you want.
You have just come across an article on the topic What is the atomic structure called?. If you found this article useful, please share it. Thank you very much.