Are you looking for an answer to the topic “What is the average atomic mass of rubidium 85 and rubidium 87?“? We answer all your questions at the website Ecurrencythailand.com in category: +15 Marketing Blog Post Ideas And Topics For You. You will find the answer right below.
Specifically, Rubidium exists as two isotopes: Rb-85 has a mass of 84.9117 amu and Rb-87 has a mass of 86.9085 amu. If the average atomic mass of rubidium is 85.4678 amu, determine the relative abundance of each isotope.Rb is 72.2% and the abundance of ‘Rb is 27.8%, what is the average atomic mass of rubidium? 237.9783 පර් Titanium has five common isotopes: 46Ti (8.0%), 47Ti (7.8%), 48Ti (73.4%), 49Ti (5.5%), 50Ti (5.3%).This is the case because the abundances of the two elements must add up to give 100% , or 1 as a decimal abundance. You know that the average atomic mass of rubidium is 85.5 u , and that the two isotopes have atomic masses equal to 85 u and 87 u , respectively.
| Isotope | Mass | Abundance |
|---|---|---|
| 85Rb | 84.911794 | 72.17% |
| 87Rb | 86.909187 | 27.83% |
| ChEBI Name | rubidium-87 atom |
|---|---|
| ChEBI ID | CHEBI:52459 |
| Definition | The stable isotope of rubidium with relative atomic mass 86.909184, 27.9 atom percent natural abundance and nuclear spin 3/2. |
| Stars | This entity has been manually annotated by the ChEBI Team. |
What is the average atomic mass of rubidium if the abundance of Rb-85 is 72.2% and Rb-87 is 27.8% with their relative mass of 85 amu and 87 amu?
Rb is 72.2% and the abundance of ‘Rb is 27.8%, what is the average atomic mass of rubidium? 237.9783 පර් Titanium has five common isotopes: 46Ti (8.0%), 47Ti (7.8%), 48Ti (73.4%), 49Ti (5.5%), 50Ti (5.3%).
What is the atomic mass of rubidium 85?
| Isotope | Mass | Abundance |
|---|---|---|
| 85Rb | 84.911794 | 72.17% |
| 87Rb | 86.909187 | 27.83% |
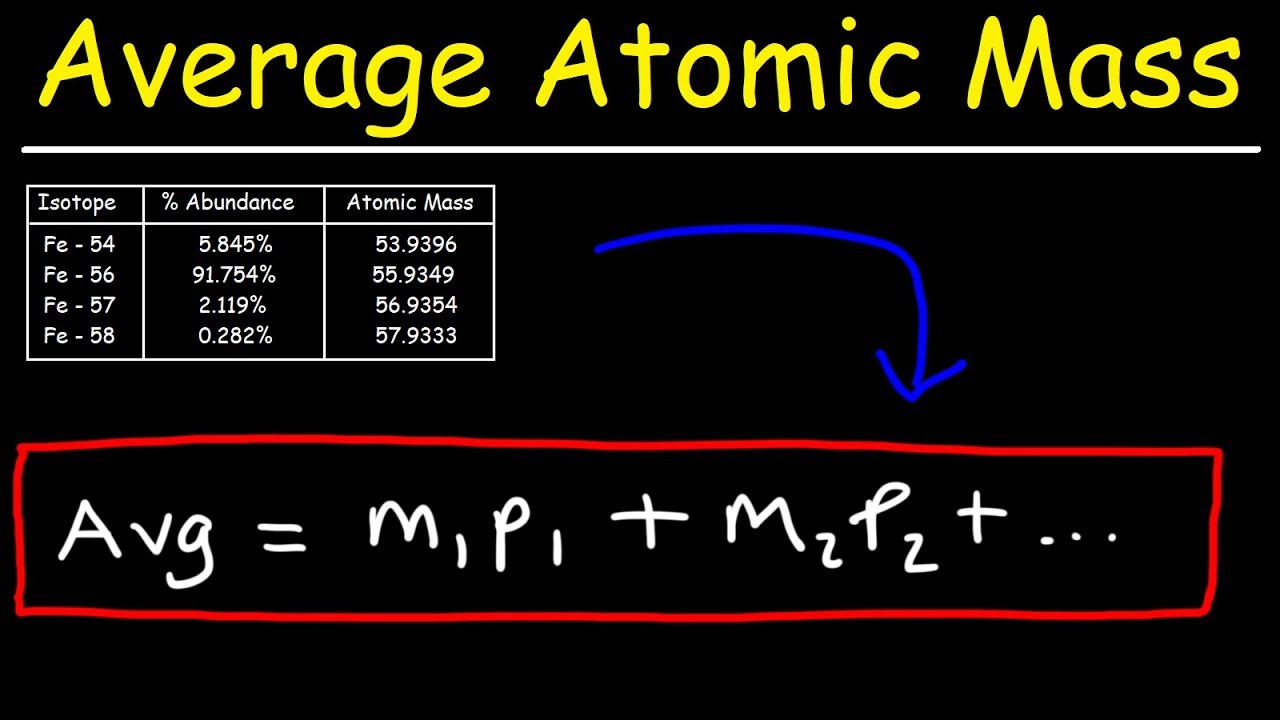
How To Calculate The Average Atomic Mass
Images related to the topicHow To Calculate The Average Atomic Mass

What is the atomic mass of 87 RB?
| ChEBI Name | rubidium-87 atom |
|---|---|
| ChEBI ID | CHEBI:52459 |
| Definition | The stable isotope of rubidium with relative atomic mass 86.909184, 27.9 atom percent natural abundance and nuclear spin 3/2. |
| Stars | This entity has been manually annotated by the ChEBI Team. |
Why is the average atomic mass of rubidium so much closer to 85 amu than 87 amu?
This is the case because the abundances of the two elements must add up to give 100% , or 1 as a decimal abundance. You know that the average atomic mass of rubidium is 85.5 u , and that the two isotopes have atomic masses equal to 85 u and 87 u , respectively.
What is the percent abundance of rubidium 85 and 87?
The relative abundance of two rubidium isotopes of atomic weight 85 and 87 are 75% and 25% respectively.
How do we calculate average atomic mass?
To calculate the average atomic mass, multiply the fraction by the mass number for each isotope, then add them together. Whenever we do mass calculations involving elements or compounds (combinations of elements), we always use average atomic masses.
What is the most common isotope of rubidium-85 or 87?
The element has two naturally occurring isotopes. Rubidium-85 is the dominant form, accounting for 72 per cent of the total, while most of the remainder is the radioactive rubidium-87, which has a half-life of 50 billion years.
See some more details on the topic What is the average atomic mass of rubidium 85 and rubidium 87? here:
Atomic Weight of Rubidium
Rubidium. Isotope. Atomic mass (Da) … 87Rb is β– active with a half-life of 48.8(5) Ga, which leaves Ar(Rb) unaffected at the currently given precision of …
CHEM 1A: Challenge Problem Set 2 – Canvas by Instructure
Rubidium has two naturally occurring isotopes, Rubidium-85 (relative mass … If rubidium has an average atomic mass of 85.47 amu, what is the abundance of.
What is the atomic number of mass number 85?
Astatine is a chemical element with the symbol At and atomic number 85.
What element has the mass number of 85?
| Atomic Mass | Name chemical element | number |
|---|---|---|
| 209 | Polonium | 84 |
| 210 | Astatine | 85 |
| 222 | Radon | 86 |
| 223 | Francium | 87 |
How many neutrons does RB 87 have?
| Properties of Rubidium-87 Isotope: | RUBIDIUM-87 |
|---|---|
| Neutron Number (N) | 50 |
| Atomic Number (Z) | 37 |
| Mass Number (A) | 87 |
| Nucleon Number (A) | 87 |
F.3 Chemistry Unit 5 (Part 4)
Images related to the topicF.3 Chemistry Unit 5 (Part 4)

What is the number of protons of rubidium 85?
| Properties of Rubidium-85 Isotope: | RUBIDIUM-85 |
|---|---|
| Atomic Number (Z) | 37 |
| Mass Number (A) | 85 |
| Nucleon Number (A) | 85 |
| Proton Number (Z) | 37 |
How many electrons does rubidium 85?
The nucleus consists of 37 protons (red) and 48 neutrons (blue). 37 electrons (green) bind to the nucleus, with a single, relatively unstable electron in the outer shell (ring).
How do you calculate average atomic mass from percent abundance?
Step 1: List the known and unknown quantities and plan the problem. Change each percent abundance into decimal form by dividing by 100. Multiply this value by the atomic mass of that isotope. Add together for each isotope to get the average atomic mass.
How do you solve for percent abundance?
To calculate the percent abundance of each isotope in a sample of an element, chemists usually divide the number of atoms of a particular isotope by the total number of atoms of all isotopes of that element and then multiply the result by 100.
How do you find the percent abundance of mass?
- Step 1: Calculate the Average Atomic Mass.
- Step 2: Set up the Relative Abundance Problem.
- Note: The sum of these two isotopes equals 100% of all the elements found in nature. …
- Step 3: Determine the Relative Abundance of the Unknown Isotope by solving for x.
How can I calculate average?
Average This is the arithmetic mean, and is calculated by adding a group of numbers and then dividing by the count of those numbers. For example, the average of 2, 3, 3, 5, 7, and 10 is 30 divided by 6, which is 5.
What do you mean by average atomic mass?
The average atomic mass (sometimes called atomic weight) of an element is the weighted average mass of the atoms in a naturally occurring sample of the element. Average masses are generally expressed in unified atomic mass units (u), where 1 u is equal to exactly one-twelfth the mass of a neutral atom of carbon-12.
What information is needed to calculate the average atomic mass of an element?
The average atomic mass for an element is calculated by summing the masses of the element’s isotopes, each multiplied by its natural abundance on Earth. When doing any mass calculations involving elements or compounds, always use average atomic mass, which can be found on the periodic table.
Average Atomic Mass (Problems 1-3)
Images related to the topicAverage Atomic Mass (Problems 1-3)

What is the daughter isotope of rubidium 87?
| Parent Isotope | Stable Daughter Product | Currently Accepted Half-Life Values |
|---|---|---|
| Thorium-232 | Lead-208 | 14.0 billion years |
| Rubidium-87 | Strontium-87 | 48.8 billion years |
| Potassium-40 | Argon-40 | 1.25 billion years |
| Samarium-147 | Neodymium-143 | 106 billion years |
Is the daughter isotope of rubidium 87 stable?
…
Rubidium-Strontium method.
| Abundance (atom%) | ||
|---|---|---|
| Mass | Rubidium | Strontium |
| 86 | 9.87 (stable) | |
| 87 | 27.83 (radioactive) | 7.00 (stable)∗ |
| 88 | 82.57 (stable) |
Related searches to What is the average atomic mass of rubidium 85 and rubidium 87?
- what is the average atomic mass of rubidium 85 and rubidium 87
- atomic mass of rubidium 87
- uranium has three common isotopes of the abundance
- how are isotopes used to determine average atomic mass?
- average atomic mass of uranium isotopes
- atomic mass of rubidium-87
- titanium has five common isotopes calculate the average atomic mass of titanium
- calculate the average atomic mass of rubidium. rubidium has two isotopes
- uranium has three common isotopes. of the abundance
- rubidium 87 percent abundance
- percent abundance of rubidium 85 and 87
- calculate the average atomic mass of rubidium rubidium has two isotopes
- a certain sample of rubidium has just two isotopes
Information related to the topic What is the average atomic mass of rubidium 85 and rubidium 87?
Here are the search results of the thread What is the average atomic mass of rubidium 85 and rubidium 87? from Bing. You can read more if you want.
You have just come across an article on the topic What is the average atomic mass of rubidium 85 and rubidium 87?. If you found this article useful, please share it. Thank you very much.