Are you looking for an answer to the topic “What is the effect of change of temperature on the solubility of A?“? We answer all your questions at the website Ecurrencythailand.com in category: +15 Marketing Blog Post Ideas And Topics For You. You will find the answer right below.
An increase in temperature puts a stress on the equilibrium condition and causes it to shift to the right. The stress is relieved because the dissolving process consumes some of the heat. Therefore, the solubility (concentration) increases with an increase in temperature.Solubility of the most of the salt rises with increase in temperature. This is because with increase in temperature, kinetic energy of the molecules increases and the solvent molecules break apart the solute molecules that are held together by intermolecular attractions more effectively.The solubility of salt increases with temperature.
What is the effect of change of a temperature on the solubility of a salt?
Solubility of the most of the salt rises with increase in temperature. This is because with increase in temperature, kinetic energy of the molecules increases and the solvent molecules break apart the solute molecules that are held together by intermolecular attractions more effectively.
What is the effect of change of temperature on the solubility of salt class 9th?
The solubility of salt increases with temperature.
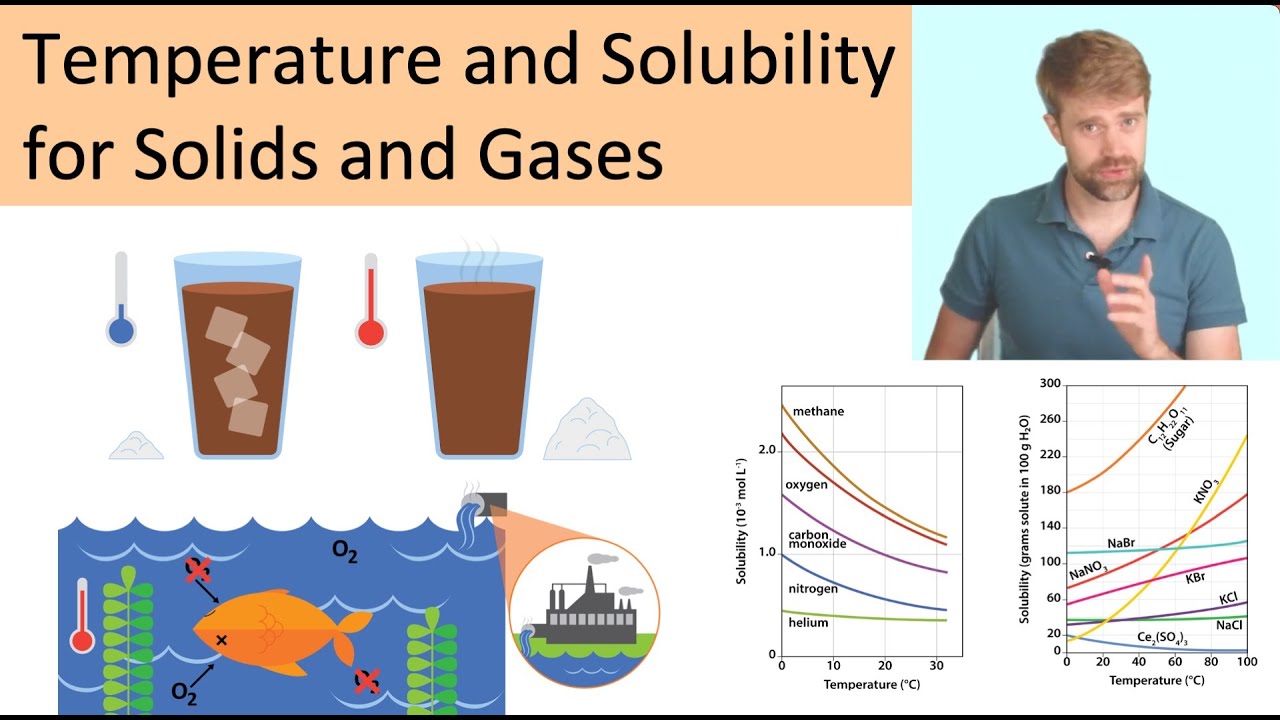
Temperature and Solubility: Solids and Gases
Images related to the topicTemperature and Solubility: Solids and Gases

What is the effect of temperature on solubility?
In general, solids become more soluble as the temperature increases. This is why sugar dissolves better in hot water than in cold water. The table shows three examples of the solubility (g of solute per 100 g water) of substances at different temperatures.
What is the effect of change of temperature on the solubility in liquid?
The solubility of most solid or liquid solutes increases with increasing temperature. The components of a mixture can often be separated using fractional crystallization, which separates compounds according to their solubilities.
What is the effect of temperature in the solubility of salt in water Brainly?
(d) The solubility of most of the salts increases in with increase in temperature. But for same salts like sodium chloride there is no effect of temperature on the solubility.
What is the effect of temperature and pressure on solubility?
The solubility of a solid may increase or decrease with increasing temperature, whereas the solubility of a gas decreases with an increase in temperature and a decrease in pressure.
What is the effect of temperature on solubility class 6?
The solubility of a substance in water increases on increasing the temperature. Larger amount of a substance can be dissolved in a given amount of water on heating it. The solubility of a substance decreases on lowering the temperature.
See some more details on the topic What is the effect of change of temperature on the solubility of A? here:
How does solubility change with temperature changes?
Usually, increasing the temperature increases the solubility of solids and liquids. Increasing the temperature always decreases the solubility of gases.
13.4 Effects of Temperature and Pressure on Solubility
The solubility of most solid or liquid solutes increases with increasing temperature. The components of a mixture can often be separated using fractional …
Effect of Temperature on Solubility — Overview & Examples
The solubility of a substance can change with temperature. At higher temperatures, most solids are more soluble, while gases are less soluble.
What is the effect of temperature on solubility of a gas? – Toppr
The solubility of a gas decreases with rising temperatures. According to Charle’s law, as we increase temperature, the volume of a given mass of gas dissolved …
What is effect of temperature on solubility class 12?
When the substance dissolves in water by an endothermic process, that is, with the absorption of heat, its solubility increases with an increase of temperature.
What is effect of temperature?
Temperature has a direct effect on whether a substance exists as a solid, liquid or gas. Generally, increasing the temperature turns solids into liquids and liquids into gases; reducing it turns gases into liquids and liquids into solids.
Effect of temperature on solubility | how dissolution affects solubility of solute
Images related to the topicEffect of temperature on solubility | how dissolution affects solubility of solute

Why solubility decreases with increase in temperature?
Increasing temperature introduces more heat into the system. Following Le Chatelier’s Principle, the system will adjust to this excess heat energy by inhibiting the dissolution reaction. Increasing temperature, therefore, decreases the solubility of the solute.
How the temperature can affect in solubility of solid and gases?
Sparingly soluble solid or liquid substances can be dissolved completely by increasing the temperature. But in case of gaseous substance, temperature inversely influences solubility i.e. as the temperature increases gases expand and escapes from their solvent.
What affects solubility?
Solubility is the maximum amount of a substance that will dissolve in a given amount of solvent at a specific temperature. There are two direct factors that affect solubility: temperature and pressure. Temperature affects the solubility of both solids and gases, but pressure only affects the solubility of gases.
What is the effect of particle size in the solubility of salt in water?
The disjoining pressure of small particles is greater than that of large particles, so small particles have a higher interfacial solubility. Due to their higher differential concentration, thinner diffusion layer,27 and increased surface area, small particles dissolve faster (Figure 8A).
What effect does stirring the solution do on the speed of dissolving the crystals?
Stirring a solute into a solvent speeds up the rate of dissolving because it helps distribute the solute particles throughout the solvent.
How does the rate of stirring affect the solubility of the solutions?
Breaking a solute into smaller pieces increases its surface area and increases its rate of solution. Stirring — With liquid and solid solutes, stirring brings fresh portions of the solvent in contact with the solute. Stirring, therefore, allows the solute to dissolve faster.
What is the effect of pressure on solubility?
Q. Solubility of gases increases with increase in pressure.
The Effect of Temperature on Solubility
Images related to the topicThe Effect of Temperature on Solubility

How do temperature and pressure affect the solubility of solids and gases in water quizlet?
For the solubility of gases in liquids, as pressure increases, solubility increases. For the solubility of solids in liquids, as temperature increases, solubility increases. For the solubility of solids in liquids, pressure has no effect.
Why does an increase in temperature usually increase solubility?
The addition of more heat facilitates the dissolving reaction by providing energy to break bonds in the solid. This is the most common situation where an increase in temperature produces an increase in solubility for solids.
Related searches to What is the effect of change of temperature on the solubility of A?
- what is the effect of change of temperature on the solubility of salt
- effect of temperature and pressure on solubility
- what is the effect of temperature on solubility of gas in liquid
- effect of pressure on solubility
- what is the effect of change of temperature on the solubility of a substance
- what is the effect of change of temperature on the solubility of a salt
- solubility is as temperature is increases
- what is the effect of change of temperature on the solubility of a salt class 9
- how does pressure affect the solubility of a gas
- solubility of gas in liquid decreases with the rise in temperature
Information related to the topic What is the effect of change of temperature on the solubility of A?
Here are the search results of the thread What is the effect of change of temperature on the solubility of A? from Bing. You can read more if you want.
You have just come across an article on the topic What is the effect of change of temperature on the solubility of A?. If you found this article useful, please share it. Thank you very much.