Are you looking for an answer to the topic “What is the effect of temperature in the solubility of a solid what is its effect in the solubility of a gas?“? We answer all your questions at the website Ecurrencythailand.com in category: +15 Marketing Blog Post Ideas And Topics For You. You will find the answer right below.
The solubility of a solid may increase or decrease with increasing temperature, whereas the solubility of a gas decreases with an increase in temperature and a decrease in pressure.The solubility of most substances depends strongly on the temperature and, in the case of gases, on the pressure. The solubility of most solid or liquid solutes increases with increasing temperature.The solubility of gases in liquids decreases with increasing temperature. Conversely, adding heat to the solution provides thermal energy that overcomes the attractive forces between the gas and the solvent molecules, thereby decreasing the solubility of the gas; pushes the reaction in Equation 4 to the left.
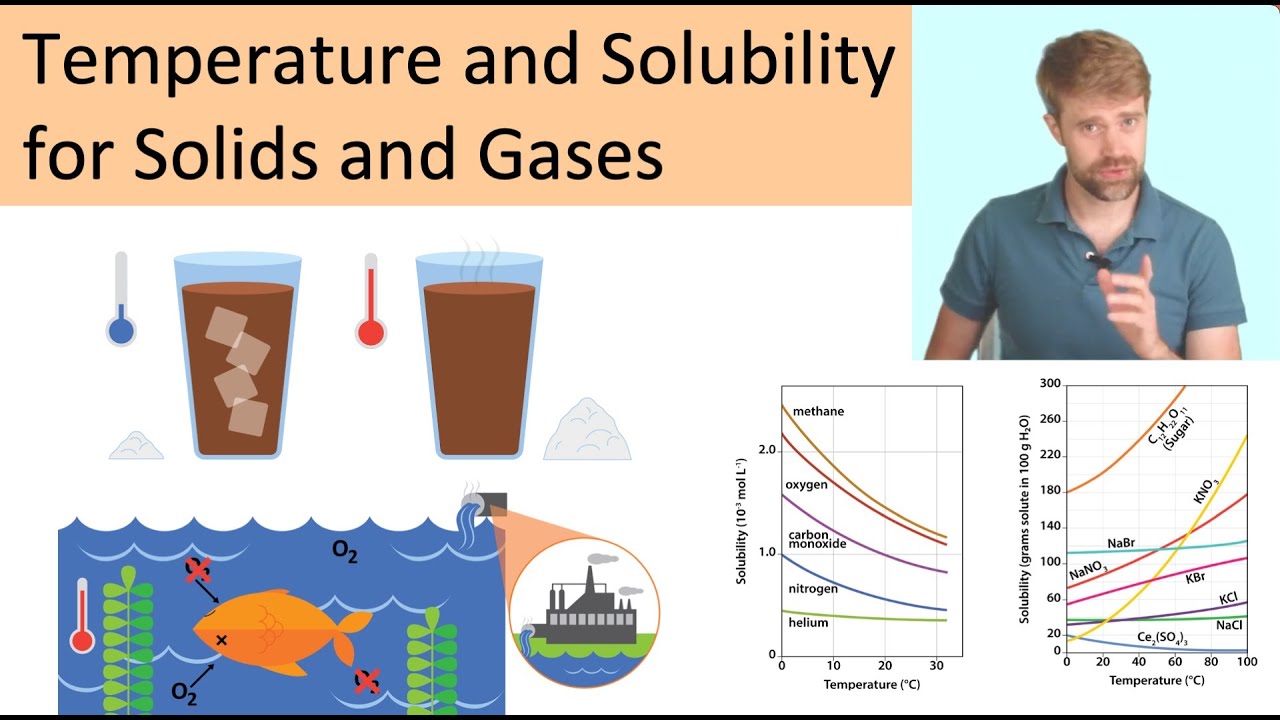
What is the effect of temperature on solubility of solid?
The solubility of most substances depends strongly on the temperature and, in the case of gases, on the pressure. The solubility of most solid or liquid solutes increases with increasing temperature.
What is the effect of temperature on the solubility of a gas?
The solubility of gases in liquids decreases with increasing temperature. Conversely, adding heat to the solution provides thermal energy that overcomes the attractive forces between the gas and the solvent molecules, thereby decreasing the solubility of the gas; pushes the reaction in Equation 4 to the left.
Temperature and Solubility: Solids and Gases
Images related to the topicTemperature and Solubility: Solids and Gases

What is the effect of temperature on a solid?
Temperature has a direct effect on whether a substance exists as a solid, liquid or gas. Generally, increasing the temperature turns solids into liquids and liquids into gases; reducing it turns gases into liquids and liquids into solids.
What is the effect of solubility of solid?
With solids, generally the solubility increases with increasing temperature. With gases, the solubility tends to decrease with increasing temperature. Pressure only affects the solubility of gases.
What is the effect of temperature on solubility class 6?
The solubility of a substance in water increases on increasing the temperature. Larger amount of a substance can be dissolved in a given amount of water on heating it. The solubility of a substance decreases on lowering the temperature.
What is the effect of temperature on solubility of solid in liquid Class 12?
Temperature has a marked effect on the solubility of a solid in a solvent. The solubility may increase or decrease with increase in with temperature. It is observed that most ionic and molecular solids become more soluble at higher temperature.
What is solubility explain the effect of temperature and pressure on the solubility of gases in liquids?
A solution in which no more solute can dissolve in the solvent at a given temperature and pressure is said to be a saturated solution as the solution contains the maximum amount of solute. The concentration of solute in such a solution is called its solubility at that temperature and pressure.
See some more details on the topic What is the effect of temperature in the solubility of a solid what is its effect in the solubility of a gas? here:
Solubility
As the temperature increases, the solubility of a gas decreases as shown by the downward trend in the graph. For solid or liquid solutes: CASE I: Decrease in …
How does solubility change with temperature changes?
Usually, increasing the temperature increases the solubility of solids and liquids. Increasing the temperature always decreases the solubility of gases.
Temperature/Pressure on Solubility – Chemistry@Elmhurst
More gas is present in a solution with a lower temperature compared to a solution with a higher temperature. The reason for this gas solubility relationship …
Effect of Temperature on Solubility — Overview & Examples
The solubility of a substance can change with temperature. At higher temperatures, most solids are more soluble, while gases are less soluble.
How do temperature and pressure affect the solubility of solids and gases in water?
The solubility of a solid may increase or decrease with increasing temperature, whereas the solubility of a gas decreases with an increase in temperature and a decrease in pressure.
How do the solubility of a solid and gas gets affected by a increase in temperature B increase in pressure?
(a) Solubility of a solid solute generally increases with an increase in temperature. This makes it possible to prepare supersaturated solutions. Solubility of a gas decreases with an increase in temperature. (b) Pressure has practically no effect on the solubility of a solid (solute) in water.
What is the effect of temperature on a matter Class 9?
Change of state of matter due to effect of temperature
We are already aware of the fact that kinetic energy of the particles of a matter increases with the increase in temperature. Due to this increase in kinetic energy the particles start to vibrate with greater speed.
What is the effect of temperature on a matter Class 9 short answer?
Effect of change of temperature on matter: On increasing the temperature of solids, the kinetic energy of the particles increases. Due to the increase in kinetic energy, the particles start vibrating with greater speed. The energy supplied by heat overcomes the forces of attraction between the particles.
How Temperature Affects the Solubility of Gases – Experiment
Images related to the topicHow Temperature Affects the Solubility of Gases – Experiment

What is the effect of temperature on different states of matter Class 9th?
The increase in temperature increases the kinetic energy of particles and inter-space between them increases. The increase in kinetic energy and space between the particles the force of attraction between particles decreases.
What affects solubility of gas?
There are two direct factors that affect solubility: temperature and pressure. Temperature affects the solubility of both solids and gases, but pressure only affects the solubility of gases.
Why does solubility of gas decrease with temperature?
As the kinetic energy of the gaseous solute increases, its molecules have a greater tendency to escape the attraction of the solvent molecules and return to the gas phase. Therefore, the solubility of a gas decreases as the temperature increases.
How does solubility of a solid in water change with temperature?
The solubility of a solid solute in a liquid solvent increases with increase in temperature.
What is the effect of temperature on solubility class 9?
Effect of the temperature on solubility
i.e. At high temperature the solubility of a solution is high so it is able to dissolve more solute, but when it is cooled, the solubility of the solution decreases and due to which the solute separate out as solid.
What is the effect of change of temperature on the solubility of salt Class 9?
The solubility of salt increases with temperature.
What is solubility class 12th?
Solubility is a physical property of a solution. It can be defined as the measure of maximum amount of solute that can be dissolved in a quantified amount of solvent. 10g of glucose powder soluble in 90 g of water.
What is the effect of temperature on the solubility of a solid in a liquid B gas in a liquid?
Increase in temperature decreases the solubility of gases in liquids. The solubility of solids increases with increase of temperature.
What is the effect of temperature and pressure on solubility of gas in liquid Class 12?
Solution : Solubility of gas decreases with increase in temperature and increases with increase in pressure.
Temperature and Gas Solubility
Images related to the topicTemperature and Gas Solubility

What is the effect of temperature on solubility of a solute explain with example?
For many solids dissolved in liquid water, the solubility increases with temperature. The increase in kinetic energy that comes with higher temperatures allows the solvent molecules to more effectively break apart the solute molecules that are held together by intermolecular attractions.
What is the effect of pressure on solubility of gases explain with an example?
With an increase in temperature, the solubility of a gas in water decreases. For example, the solubility of carbon dioxide in water under normal atmospheric pressure is low, but when the water surface is subjected to higher pressure, a lot more of CO2 gas gets dissolved in it.
Related searches to What is the effect of temperature in the solubility of a solid what is its effect in the solubility of a gas?
- solubility of gases in water
- what is the effect of temperature on the solubility of a solid in a liquid
- what is the effect of temperature on the solubility of gases in liquids?
- factors affecting solubility
- how does temperature affect solubility
- what is the effect of pressure on the solubility of gases in liquids
- effect of pressure on solubility of solid in liquid
- what is the effect of temperature on the solubility of gases in liquids
- what is the effect of pressure on the solubility of gases in liquids?
- solubility of gas in liquid decreases with the rise in temperature
Information related to the topic What is the effect of temperature in the solubility of a solid what is its effect in the solubility of a gas?
Here are the search results of the thread What is the effect of temperature in the solubility of a solid what is its effect in the solubility of a gas? from Bing. You can read more if you want.
You have just come across an article on the topic What is the effect of temperature in the solubility of a solid what is its effect in the solubility of a gas?. If you found this article useful, please share it. Thank you very much.