Are you looking for an answer to the topic “What is the effect of temperature on Henry’s law constant and on solubility of a gas on liquid?“? We answer all your questions at the website Ecurrencythailand.com in category: +15 Marketing Blog Post Ideas And Topics For You. You will find the answer right below.
1 Answer. Solubility of a gas in liquid decreases with increase in temperature. KH value increases with the increase in temperature.The volume of given mass of dissolved gas in solution also increases with increase of temperature. It becomes impossible for solvent to accommodate gaseous solute in it and gas bubbles out. Hence, with increase in temperature, the solubility of a gas in a liquid decreases.The solubility of gases in liquids decreases with increasing temperature. Conversely, adding heat to the solution provides thermal energy that overcomes the attractive forces between the gas and the solvent molecules, thereby decreasing the solubility of the gas; pushes the reaction in Equation 4 to the left.

What is the effect of temperature on Henry’s law constant for the solubility of a gas in a solvent keeping the pressure of gas same?
The volume of given mass of dissolved gas in solution also increases with increase of temperature. It becomes impossible for solvent to accommodate gaseous solute in it and gas bubbles out. Hence, with increase in temperature, the solubility of a gas in a liquid decreases.
What is the effect of temperature on the solubility of gas in a liquid?
The solubility of gases in liquids decreases with increasing temperature. Conversely, adding heat to the solution provides thermal energy that overcomes the attractive forces between the gas and the solvent molecules, thereby decreasing the solubility of the gas; pushes the reaction in Equation 4 to the left.
Pressure and Gas Solubility (Henry’s Law)
Images related to the topicPressure and Gas Solubility (Henry’s Law)

How does temperature affect Henry’s constant?
KH depends only on nature of gas, nature of liquid and temperature (T). As temperature increases, ‘KH ‘ increases and X decreases.
What is the effect of temperature on solubility of gas in a liquid give reason Class 12?
Hence, solubility of gas in liquid decreases with increase in temperature.
What is the effect of temperature on solubility?
In general, solids become more soluble as the temperature increases. This is why sugar dissolves better in hot water than in cold water. The table shows three examples of the solubility (g of solute per 100 g water) of substances at different temperatures.
What is the effect of temperature on solubility class 9?
Effect of the temperature on solubility
i.e. At high temperature the solubility of a solution is high so it is able to dissolve more solute, but when it is cooled, the solubility of the solution decreases and due to which the solute separate out as solid.
What is the effect of temperature on the solubility of gases in liquids Class 11?
Solubility of gases in liquids decreases with increase in temperature.
See some more details on the topic What is the effect of temperature on Henry’s law constant and on solubility of a gas on liquid? here:
State Henry’s law. What is the effect of temperature on … – Toppr
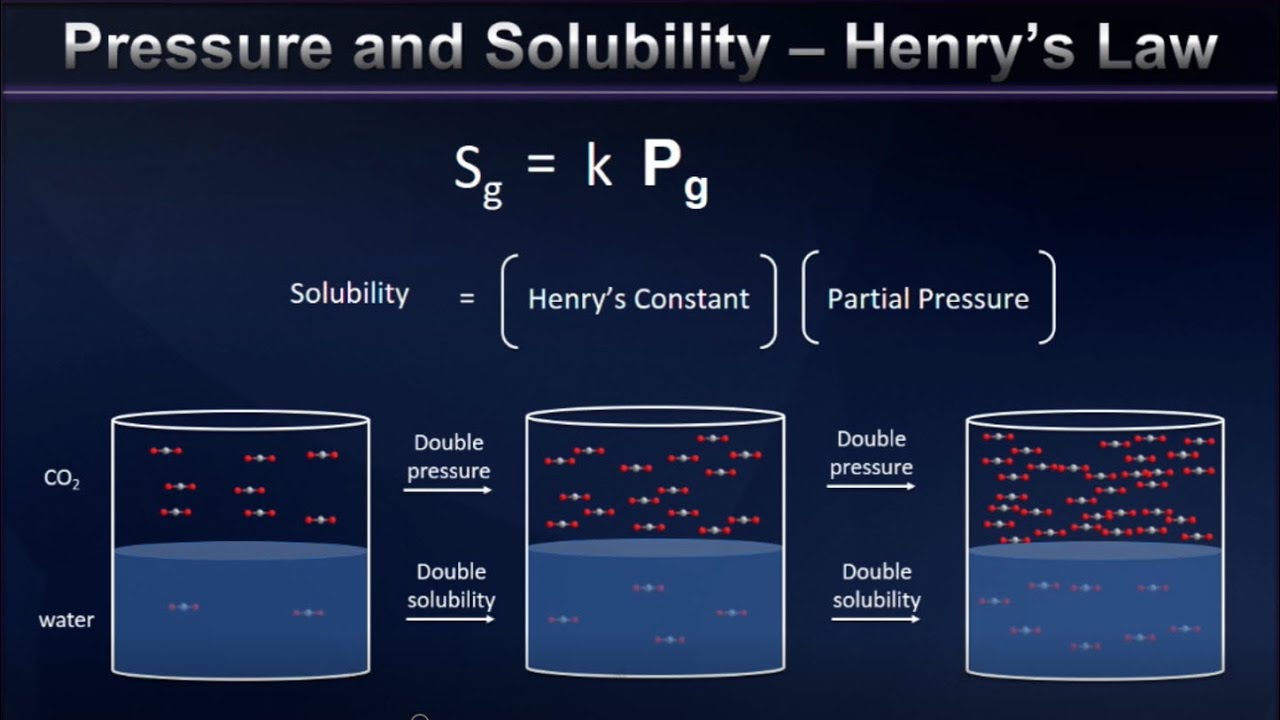
Henry’s law states that the solubility of a gas in a liquid at constant temperature is proportional to the pressure of the gas above the solution.
13.4: Effects of Temperature and Pressure on Solubility
The solubility of gases in liquids decreases with increasing …
State Henry’S Law. What is the Effect of Temperature on the …
The solubility of gases is dependent on temperature. An increase in temperature results in a decrease in gas solubility in liquid, while a decrease in …
What is the effect of temperature on Henry’s law constant KH …
Solubility of a gas in liquid decreases with increase in temperature. KH value increases with the increase in temperature. Related Answer. Henry’s law Is …
What is Henry Law of solubility?
In physical chemistry, Henry’s law is a gas law that states that the amount of dissolved gas in a liquid is proportional to its partial pressure above the liquid.
What happens to the solubility of a gas in a liquid at constant temperature when pressure get increased?
The solubility of the gas in a liquid solution decreases with increase in temperature. This is because, with an increase in temperature, the kinetic energy of the gas molecule increases, thus it becomes difficult for liquid molecules to hold them.
Henry’s Law Explained – Gas Solubility Partial Pressure – Chemistry Problems
Images related to the topicHenry’s Law Explained – Gas Solubility Partial Pressure – Chemistry Problems

What is the relation between Henry’s constant and solubility?
Henry’s constant increases with an increase in the temperature. Therefore, the solubility of the gas decreases. Increasing the pressure increases the solubility and increase in temperature decreases the solubility of the gas in the liquid.
What affects Henry’s law constant?
The value of the Henry’s law constant of a gas is dependent on the following factors: The nature of the gas. The nature of the solvent. Temperature & pressure.
What does Henry’s law constant depends on?
It is important to keep in mind that Henry’s law constants are highly dependent on temperature, since both vapor pressure and solubility are also temperature dependent. So, when using published KH values, one must compare them isothermically.
What is the effect of temperature on I solubility of solid in liquid II solubility of gas in liquid also give reason?
As the temperature increases, the particles of the solid move faster, which increases the chances that they will interact with more of the solvent particles. This results in increasing the rate at which a solution occurs. For all gases, as the temperature increases, solubility decreases.
What is solubility explain the effect of temperature and pressure on the solubility of gases in liquids?
A solution in which no more solute can dissolve in the solvent at a given temperature and pressure is said to be a saturated solution as the solution contains the maximum amount of solute. The concentration of solute in such a solution is called its solubility at that temperature and pressure.
How the temperature can affect in solubility of solid and gases?
Sparingly soluble solid or liquid substances can be dissolved completely by increasing the temperature. But in case of gaseous substance, temperature inversely influences solubility i.e. as the temperature increases gases expand and escapes from their solvent.
What is the effect of temperature on solubility in water give example?
The effect of temperature on solubility of a substance depends on enthalpy of solution. a. When the substance dissolves in water by an endothermic process, that is, with the absorption of heat, its solubility increases with an increase of temperature. e.g. KCl dissolve in water by endothermic process.
Henry’s Law and Gas Solubility Explained
Images related to the topicHenry’s Law and Gas Solubility Explained
What is the effect of temperature on solubility class 12?
Hence, we can say that increasing the temperature increases the solubility of solids and liquids, whereas in case of gases increase in temperature decreases the solubility of any substance.
What is the effect of temperature on solubility class 6?
The solubility of a substance in water increases on increasing the temperature. Larger amount of a substance can be dissolved in a given amount of water on heating it. The solubility of a substance decreases on lowering the temperature.
Related searches to What is the effect of temperature on Henry’s law constant and on solubility of a gas on liquid?
- does henrys law constant change with temperature
- how does an increase in pressure affect the solubility of a solid or liquid
- henry’s law constant for oxygen in water at different temperatures
- does henry’s law constant change with temperature
- what is the effect of temperature on the solubility of a gas
- henrys law constant for oxygen in water at different temperatures
- how does an increase in pressure affect the solubility of a solid or liquid?
- what is the effect of temperature on solubility of solid in liquid
Information related to the topic What is the effect of temperature on Henry’s law constant and on solubility of a gas on liquid?
Here are the search results of the thread What is the effect of temperature on Henry’s law constant and on solubility of a gas on liquid? from Bing. You can read more if you want.
You have just come across an article on the topic What is the effect of temperature on Henry’s law constant and on solubility of a gas on liquid?. If you found this article useful, please share it. Thank you very much.