Are you looking for an answer to the topic “What is the effect of temperature on solubility of solid in water give examples?“? We answer all your questions at the website Ecurrencythailand.com in category: +15 Marketing Blog Post Ideas And Topics For You. You will find the answer right below.
The effect of temperature on solubility of a substance depends on
. a. When the substance dissolves in water by an
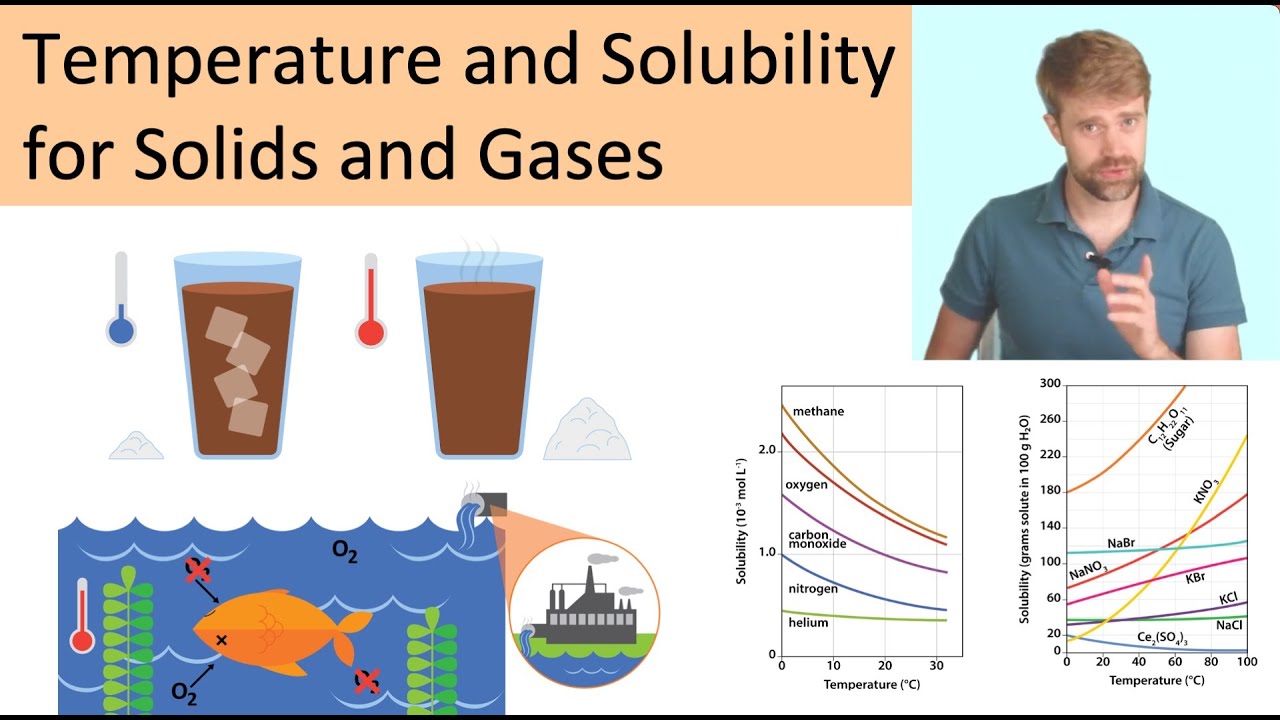
, that is, with the absorption of heat, its solubility increases with an increase of temperature. e.g. KCl dissolve in water by endothermic process.In general, solids become more soluble as the temperature increases. This is why sugar dissolves better in hot water than in cold water. The table shows three examples of the solubility (g of solute per 100 g water) of substances at different temperatures.The solubility of most solid or liquid solutes increases with increasing temperature. The components of a mixture can often be separated using fractional crystallization, which separates compounds according to their solubilities. The solubility of a gas decreases with increasing temperature.
What is the effect of temperature on solubility of solute in water?
In general, solids become more soluble as the temperature increases. This is why sugar dissolves better in hot water than in cold water. The table shows three examples of the solubility (g of solute per 100 g water) of substances at different temperatures.
What is the effect of temperature in the solubility of a solid what is its effect in the solubility of a gas?
The solubility of most solid or liquid solutes increases with increasing temperature. The components of a mixture can often be separated using fractional crystallization, which separates compounds according to their solubilities. The solubility of a gas decreases with increasing temperature.
Temperature and Solubility: Solids and Gases
Images related to the topicTemperature and Solubility: Solids and Gases

What is the effect of temperature on solubility class 9?
Effect of the temperature on solubility
i.e. At high temperature the solubility of a solution is high so it is able to dissolve more solute, but when it is cooled, the solubility of the solution decreases and due to which the solute separate out as solid.
How does temperature affect the solubility of solid substances?
The solubility of a solid in water increases with an increase in temperature.
What is the effect of temperature on solubility class 6?
The solubility of a substance in water increases on increasing the temperature. Larger amount of a substance can be dissolved in a given amount of water on heating it. The solubility of a substance decreases on lowering the temperature.
What is the effect of temperature on solubility of solid in liquid Class 12?
Temperature has a marked effect on the solubility of a solid in a solvent. The solubility may increase or decrease with increase in with temperature. It is observed that most ionic and molecular solids become more soluble at higher temperature.
What is solubility explain the effect of temperature and pressure on the solubility of gases in liquids?
A solution in which no more solute can dissolve in the solvent at a given temperature and pressure is said to be a saturated solution as the solution contains the maximum amount of solute. The concentration of solute in such a solution is called its solubility at that temperature and pressure.
See some more details on the topic What is the effect of temperature on solubility of solid in water give examples? here:
Solid Solubility and Temperature | Introduction to Chemistry
For many solids dissolved in liquid water, the solubility increases with temperature. The increase in kinetic energy that comes with higher temperatures …
13.4 Effects of Temperature and Pressure on Solubility
Although the solubility of a solid generally increases with increasing temperature, there is no simple relationship between the structure of a substance and the …
Solubility
Gases dissolve in liquids to form solutions. This dissolution is an equilibrium process for which an equilibrium constant can be written. For example, the …
Effect of Temperature on Solubility — Overview & Examples
Sugar is pretty soluble in water (the solvent of tea) however when it is cold, the sugar doesn’t dissolve as well. If heated, the molecules of the sugar would …
How do temperature and pressure affect the solubility of solids and gases in water?
The solubility of a solid may increase or decrease with increasing temperature, whereas the solubility of a gas decreases with an increase in temperature and a decrease in pressure.
The Effect of Temperature on Solubility
Images related to the topicThe Effect of Temperature on Solubility

What is the effect of pressure on the solubility of solids in liquids?
Effect of pressure on solubility of solids in liquids
Liquids and solids exhibit practically no change of solubilitywith changes in pressure. Gases as might be expected, increase in solubility with an increase in pressure.
What is the effect of temperature on solubility of gases explain with an example?
The solubility of gases in liquids decreases with increasing temperature. Conversely, adding heat to the solution provides thermal energy that overcomes the attractive forces between the gas and the solvent molecules, thereby decreasing the solubility of the gas; pushes the reaction in Equation 4 to the left.
What is the effect of temperature on solubility of salt?
Answer: Temperature does effect the solubility of any solid solute in a solution,like in the case of salt in water. the effect of temperature depends on the nature of reaction. if the reaction is an endothermic reaction,then the increase in temperature has a positive effect and the solubility increases.
What is the effect of temperature on solubility of gas in liquid Class 12?
Hence, solubility of gas in liquid decreases with increase in temperature.
Why does the solubility of solids in water increases with an increase in temperature?
The addition of more heat facilitates the dissolving reaction by providing energy to break bonds in the solid. This is the most common situation where an increase in temperature produces an increase in solubility for solids.
How do the solubility of a solid and gas gets affected by a increase in temperature B increase in pressure?
(a) Solubility of a solid solute generally increases with an increase in temperature. This makes it possible to prepare supersaturated solutions. Solubility of a gas decreases with an increase in temperature. (b) Pressure has practically no effect on the solubility of a solid (solute) in water.
What is the effect of pressure on solubility of gases explain with an example?
With an increase in temperature, the solubility of a gas in water decreases. For example, the solubility of carbon dioxide in water under normal atmospheric pressure is low, but when the water surface is subjected to higher pressure, a lot more of CO2 gas gets dissolved in it.
Effect of temperature on the solubility of a solid
Images related to the topicEffect of temperature on the solubility of a solid

What is the effect of temperature on solubility of kno3 and caso4 in water?
Solution : The solubility of potassium nitrate `(KNO_3)` in water increases with increase in temperature. <br> The solubility of calcium sulphate `(CaSO_4)` in water decreases with increase in temperature.
What is solubility explain effect of temperature on solubility?
Key Points. For many solids dissolved in liquid water, the solubility increases with temperature. The increase in kinetic energy that comes with higher temperatures allows the solvent molecules to more effectively break apart the solute molecules that are held together by intermolecular attractions.
Related searches to What is the effect of temperature on solubility of solid in water give examples?
- what is the effect of temperature on solubility of solid in water give examples
- what is the effect of temperature on solubility of kno3 and caso4 in water
- what is the effect of temperature on solubility of solid in liquid class 9
- effect of temperature and pressure on solubility
- what is the effect of temperature on the solubility of a solid in a liquid
- effect of pressure on solubility of solid in liquid
- what is the effect of temperature on solubility of a salt
- what is solubility
- what is the effect of temperature on solubility of gases in liquid
Information related to the topic What is the effect of temperature on solubility of solid in water give examples?
Here are the search results of the thread What is the effect of temperature on solubility of solid in water give examples? from Bing. You can read more if you want.
You have just come across an article on the topic What is the effect of temperature on solubility of solid in water give examples?. If you found this article useful, please share it. Thank you very much.