Are you looking for an answer to the topic “What is the effect of temperature on the rate of solubility?“? We answer all your questions at the website Ecurrencythailand.com in category: +15 Marketing Blog Post Ideas And Topics For You. You will find the answer right below.
The solubility of most substances depends strongly on the temperature and, in the case of gases, on the pressure. The solubility of most solid or liquid solutes increases with increasing temperature.Effect of the temperature on solubility
i.e. At high temperature the solubility of a solution is high so it is able to dissolve more solute, but when it is cooled, the solubility of the solution decreases and due to which the solute separate out as solid.Key Points. For many solids dissolved in liquid water, the solubility increases with temperature. The increase in kinetic energy that comes with higher temperatures allows the solvent molecules to more effectively break apart the solute molecules that are held together by intermolecular attractions.

What is the effect of temperature on solubility class 9?
Effect of the temperature on solubility
i.e. At high temperature the solubility of a solution is high so it is able to dissolve more solute, but when it is cooled, the solubility of the solution decreases and due to which the solute separate out as solid.
How does temperature affect the rate of solubility?
Key Points. For many solids dissolved in liquid water, the solubility increases with temperature. The increase in kinetic energy that comes with higher temperatures allows the solvent molecules to more effectively break apart the solute molecules that are held together by intermolecular attractions.
The Effect of Temperature on Solubility
Images related to the topicThe Effect of Temperature on Solubility

What is the effect of temperature on solubility class 6?
The solubility of a substance in water increases on increasing the temperature. Larger amount of a substance can be dissolved in a given amount of water on heating it. The solubility of a substance decreases on lowering the temperature.
What is the effect of temperature on solubility example?
Temperature and Solid Solubility
If heated, the molecules of the sugar would gain energy and move around more. This increases the solubility of the sugar in the tea. If the tea is heated and sugar is then added, the tea transfer it’s heat to the sugar helping it to dissolve.
What is the effect of temperature on solubility class 12?
Hence, we can say that increasing the temperature increases the solubility of solids and liquids, whereas in case of gases increase in temperature decreases the solubility of any substance.
What is effect of temperature?
Temperature has a direct effect on whether a substance exists as a solid, liquid or gas. Generally, increasing the temperature turns solids into liquids and liquids into gases; reducing it turns gases into liquids and liquids into solids.
Why does temperature increase solubility?
The addition of more heat facilitates the dissolving reaction by providing energy to break bonds in the solid. This is the most common situation where an increase in temperature produces an increase in solubility for solids.
See some more details on the topic What is the effect of temperature on the rate of solubility? here:
Temperature Effects on Solubility – Chemistry LibreTexts
The solubility of solutes is dependent on temperature. When a solid dissolves in a liquid, a change in the physical state of the solid …
The Effect of Temperature on the Rate of Dissolving. – Science …
Increasing the temperature increases the rate of dissolving because, at higher temperatures, the solvent molecules are moving more rapidly and therefore come …
Solubility
As the temperature increases, the solubility of a gas decreases as shown by the downward trend in the graph. For solid or liquid solutes: CASE I: Decrease in …
Factors affecting solubility and rate of solution notes.doc
Notes: FACTORS THAT AFFECT SOLUBILITY and FACTORS AFFECTING RATE OF SOLUTION … the temperature of the solution increases the solubility of a solid solute.
Why does solubility decrease with temperature?
Increasing temperature introduces more heat into the system. Following Le Chatelier’s Principle, the system will adjust to this excess heat energy by inhibiting the dissolution reaction. Increasing temperature, therefore, decreases the solubility of the solute.
Temperature and Solubility: Solids and Gases
Images related to the topicTemperature and Solubility: Solids and Gases
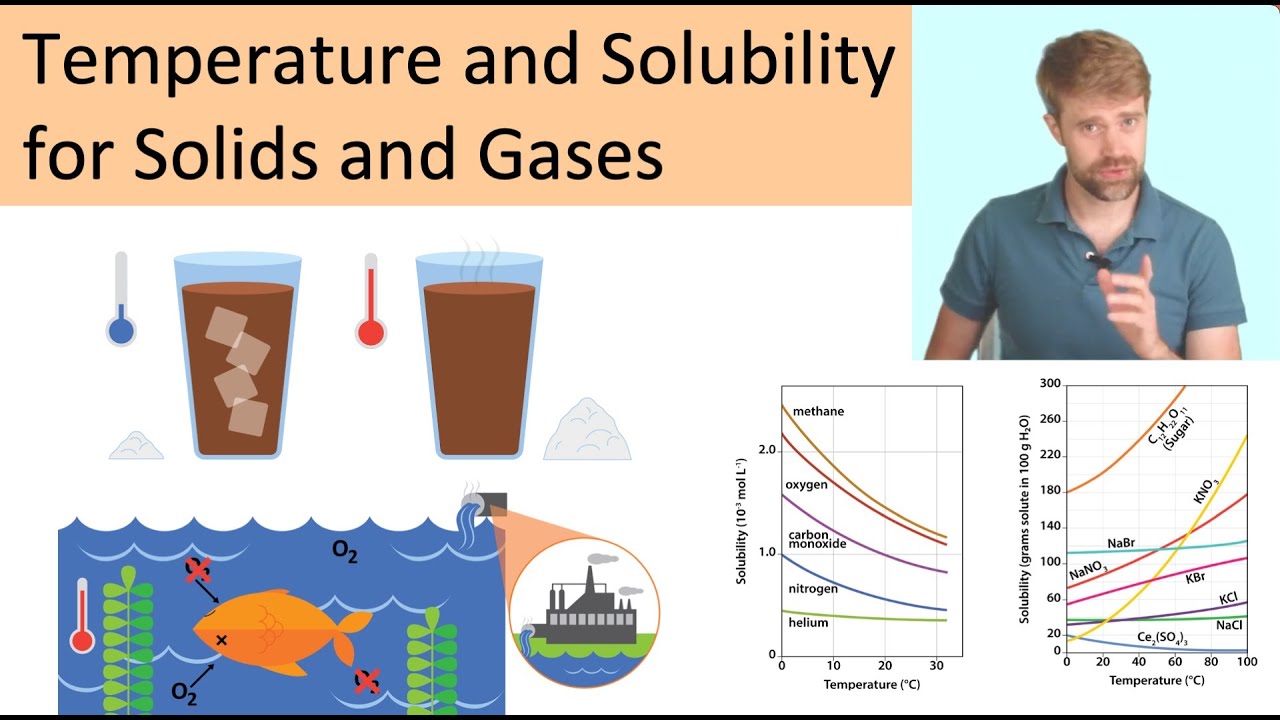
What is the effect of temperature on solubility of a gas in a liquid?
The solubility of gases in liquids decreases with increasing temperature. Conversely, adding heat to the solution provides thermal energy that overcomes the attractive forces between the gas and the solvent molecules, thereby decreasing the solubility of the gas; pushes the reaction in Equation 4 to the left.
What is solubility explain the effect of temperature and pressure?
How does temperature and pressure affect solubility? In this reaction, an increase in pressure and a rise in temperature contributes to greater solubility. To decrease the partial pressure, an increase in pressure results in more gas particles entering the liquid. The solubility will, therefore, increase.
What is the effect of temperature on the solute?
Solubility of a solute in a solvent increases with increase in temperature.
What are the factors that affects rate of solubility?
There are two direct factors that affect solubility: temperature and pressure. Temperature affects the solubility of both solids and gases, but pressure only affects the solubility of gases.
What are the 3 factors that affect solubility?
- Temperature: By changing the temperature we can increase the soluble property of a solute. …
- Forces and Bonds: Like dissolves in like. …
- Pressure: Gaseous substances are much influenced than solids and liquids by pressure.
What is the effect of temperature on the solubility of gases in liquids Class 11?
Solubility of gases in liquids decreases with increase in temperature.
Does temperature affect rate of reaction?
The rate of a chemical reaction can be changed by altering the temperature. If the temperature is increased: the reactant particles move more quickly. they have more energy.
12.4 The Effect of Temperature on Solubility
Images related to the topic12.4 The Effect of Temperature on Solubility

How does temperature affect the rate of reaction experiment?
If the temperature is raised, the kinetic energies of both A and B are increased so that there are more collisions per second, and a greater fraction of these will lead to chemical reaction. The rate, therefore, generally increases with increasing temperature.
How does temperature affect reaction rate examples?
- Cookies bake faster at higher temperatures.
- Bread dough rises more quickly in a warm place than in a cool one.
- Low body temperatures slow down metabolism. …
- Lightsticks produce light via a chemical reaction.
Related searches to What is the effect of temperature on the rate of solubility?
- effect of temperature and pressure on solubility
- what is the effect of temperature on solubility class 9
- what is the effect of temperature on solubility of gas in liquid
- explain the effect of temperature on the solubility of a salt
- solubility is as temperature is increases
- effect of pressure on solubility of solid in liquid
- what is the effect of pressure on the solubility of gases in liquids
- what is solubility
- what is the effect of temperature on the rate of solubility class 9
- what is the effect of temperature on the rate of solubility
- solubility of gas in liquid decreases with the rise in temperature
Information related to the topic What is the effect of temperature on the rate of solubility?
Here are the search results of the thread What is the effect of temperature on the rate of solubility? from Bing. You can read more if you want.
You have just come across an article on the topic What is the effect of temperature on the rate of solubility?. If you found this article useful, please share it. Thank you very much.