Are you looking for an answer to the topic “What is the electron affinity of B?“? We answer all your questions at the website Ecurrencythailand.com in category: +15 Marketing Blog Post Ideas And Topics For You. You will find the answer right below.
There are two types of electron affinity, first and second. The first involves the addition of an electron to a neutral atom. Because this exothermic process releases energy, first electron affinities are negative values. The second pertains to the addition of an electron to a negative ion.Electron affinity is the energy change that results from adding an electron to a gaseous atom. For example, when a fluorine atom in the gaseous state gains an electron to form F⁻(g), the associated energy change is -328 kJ/mol.Electron affinity is the tendency is attract shared pair of electron towards itself. Its value decreases in a group and increases in a period. The correct order of electron affinities is as follows N<O<S<Cl.
…
Elements.
| Z | 5 |
|---|---|
| Element | B |
| Name | Boron |
| Electron affinity (eV) | 0.279 723(25) |
| Electron affinity (kJ/mol) | 26.989(3) |
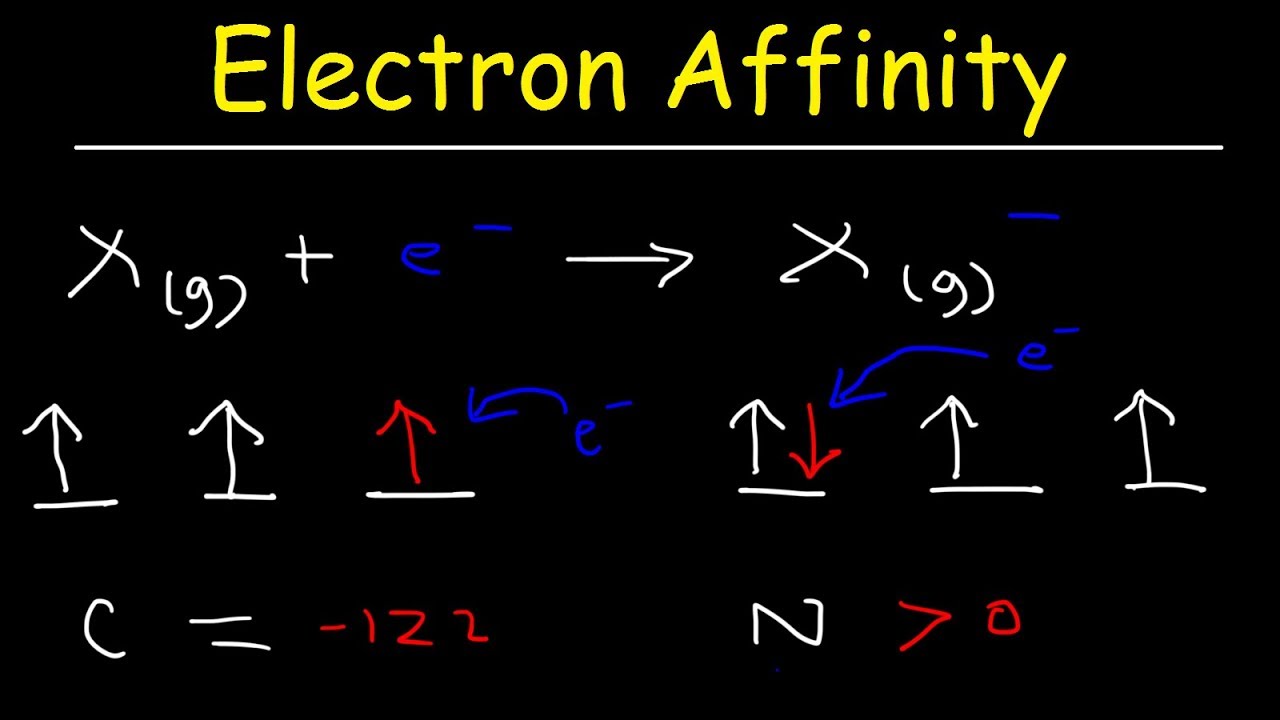
What is 1st and 2nd electron affinity?
There are two types of electron affinity, first and second. The first involves the addition of an electron to a neutral atom. Because this exothermic process releases energy, first electron affinities are negative values. The second pertains to the addition of an electron to a negative ion.
What is the value of electron affinity?
Electron affinity is the energy change that results from adding an electron to a gaseous atom. For example, when a fluorine atom in the gaseous state gains an electron to form F⁻(g), the associated energy change is -328 kJ/mol.
Electron Affinity Trend, Basic Introduction, Chemistry
Images related to the topicElectron Affinity Trend, Basic Introduction, Chemistry

What is the order of electron affinity?
Electron affinity is the tendency is attract shared pair of electron towards itself. Its value decreases in a group and increases in a period. The correct order of electron affinities is as follows N<O<S<Cl.
What is electron affinity class 11?
Electron affinity is defined as the amount of energy released when an electron is added to a neutral atom to form an anion. It is the change in the potential energy of an atom when an electron is added to a neutral gaseous atom to form a negative ion. The unit of electron affinity is KJ per mole (KJmol).
What is the first electron affinity?
Defining first electron affinity
The first electron affinity is the energy released when 1 mole of gaseous atoms each acquire an electron to form 1 mole of gaseous 1- ions. This is more easily seen in symbol terms. It is the energy released (per mole of X) when this change happens.
Why is electron affinity of CL more than F?
the electron affinity of the fluorine is less than chlorine because the size of fluorine is too small as size decreases from left to right inside period, whereas chlorine has a larger size to accommodate electrons hence electron affinity of chlorine is more than fluorine.
What has the lowest electron affinity?
The correct answer is Argon. Argon has all filled orbitals as well as a filled valence shell. As a result, it doesn’t want to lose or gain any electrons. Hence, argon has the lowest electron affinity.
See some more details on the topic What is the electron affinity of B? here:
Periodic Trends — Electron Affinity
The electron affinity of an element is the energy change which accompanies the addition of an electron to an atom in the gas phase to produce a negatively …
Ionization Energy and Electron Affinity
(a) Elements on the left side of the periodic table are more likely than those on the right to form positive ions. (b) The maximum positive charge on an ion is …
The correct order of electron affinity of B, C, N, O is : – Toppr
The correct order of electron affinity of B, C, N, O is O>C>B>N. On moving left to right in a period, the electron affinity becomes more negative. N has …
Electron Affinity – an overview | ScienceDirect Topics
The electron affinity (EA) is defined as the energy difference between the 3P ground state of the boron anion B− and the 2P ground state of the B atom.
Why 2nd and 3rd electron affinities are endothermic?
Answer: The second electron affinity is a positive value as energy is needed to add a second electron to an ion with a pre-existing negative charge. This negative charge repels the incoming electron. Adding an electron is thus an endothermic process, and requires energy input.
Which has more electron affinity O or O?
Actually, since O− is negatively-charged, it would be more difficult to accept an electron, and significant coulombic repulsion would make the electron affinity of O− more positive than that of O (which is negative).
What is Al electron affinity?
The electron affinity of aluminium is 42.5 kJ mol‑1.
Which has more electron affinity?
Halogens has higher electron affinity and it is supposed to be for fluorine, but chlorine has higher electron affinity than fluorine due to fluorine’s smaller size. Hence, among given options chlorine has highest electron affinity.
Electron affinity: period trend | Atomic structure and properties | AP Chemistry | Khan Academy
Images related to the topicElectron affinity: period trend | Atomic structure and properties | AP Chemistry | Khan Academy
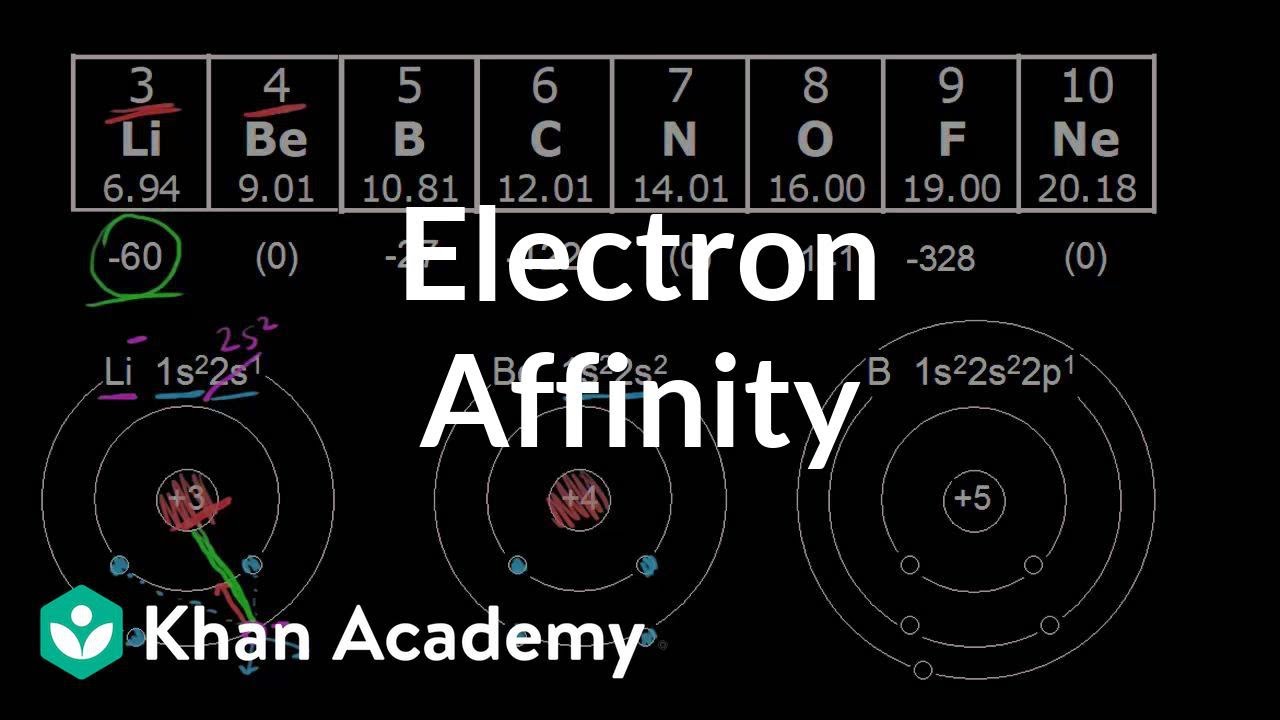
Which of the following orders for electron affinity is are correct a/s O se B Cl F c/s O d/o S?
Therefore, the correct order of electron affinity is Cl > F > Br. Hence, option C is the required answer.
Which of the following orders for electron affinity is are correct 😕
Cl>F>Br.
Why Lithium has more electron affinity than Boron?
The electron added in the Lithium goes into the 2s subshell which being close to the nucleus experiences greater electrostatic force of attraction compared to the one going into the 2p subshell of the Boron atom which is relatively more diffused( spread out in space ) .
What is electron affinity Vedantu?
Electron affinity is defined as the quantitative measurement of the energy change that results from adding a new electron to a neutral atom or molecule in the gaseous state. The more negative the electron affinity value, the higher an atom’s affinity for electrons.
Why are electron affinities negative?
Electron affinities are negative numbers because energy is released. The elements of the halogen group (Group 17) gain electrons most readily, as can be seen from their large negative electron affinities. This means that more energy is released in the formation of a halide ion than for the anions of any other elements.
What is the trend of electron affinity in the periodic table?
Electron Affinity Trend
Electron affinity generally increases moving left to right across an element period (periodic table row). The exception is the noble gases, which are in the last column of the table. Each of these elements has a completely filled valence electron shell and an electron affinity approaching zero.
What is the electron affinity of carbon?
The electron affinity of carbon is 153.9 kJ mol‑1.
What is the second electron affinity of oxygen?
The required second electron affinity of oxygen is $744.4kJ/mol$. So the correct option is D. Additional Information:-Electron affinity is defined as the minimum amount of energy released when an electron is added to its neutral atom or molecule in its gaseous state to form a negative ion.
Why is electron affinity of fluorine negative?
Electron affinity is the energy released when 1 mole of gaseous atoms each acquire an electron to form 1 mole of gaseous 1- ions. Fluorine has a negative value as energy is released when the electron is gained. This is because there is an attraction between the protons in the nucleus and the added electrons.
Which has higher electron affinity Br or Cl?
Chlorine does have a higher electron affinity than bromine.
What is electron affinity? | Chemistry | Extraclass.com
Images related to the topicWhat is electron affinity? | Chemistry | Extraclass.com

Which has high electron affinity F or Cl?
Electronegativity of fluorine is greater than that of chlorine but electron affinity of chlorine is greater than that of fluorine.
Which has more electron affinity F minus or Cl minus?
Electron affinity of Cl is greater than that of F.
Related searches to What is the electron affinity of B?
- electron affinity of c
- what is the reaction that corresponds to the electron affinity of bromine br
- nitrogen electron affinity
- what is the electron affinity of be
- electron affinity chart
- electron affinity of f
- what is the electron affinity of br
- what is the electron affinity of beryllium
- what does electron affinity do
- what is the reaction that corresponds to the electron affinity of bromine
- what is the electron affinity of bromine
- electron affinity of na
- what is the reaction that corresponds to the electron affinity of bromine br b r
- what is the reaction that corresponds to the electron affinity of bromine brbr
- what is the electron affinity of o
- what does affinity for electrons mean
- electron affinity of li
- o electron affinity
- what is the connection between ionization energy electronegativity and electron affinity of elements
- electron affinity trend
- in charging by friction the electron affinity of the material is what causes the transfer of
Information related to the topic What is the electron affinity of B?
Here are the search results of the thread What is the electron affinity of B? from Bing. You can read more if you want.
You have just come across an article on the topic What is the electron affinity of B?. If you found this article useful, please share it. Thank you very much.