Are you looking for an answer to the topic “What is the electron affinity of bromine?“? We answer all your questions at the website Ecurrencythailand.com in category: +15 Marketing Blog Post Ideas And Topics For You. You will find the answer right below.
Electron Affinity of Bromine is 324.6 kJ/mol.When electron affinity is negative, it implies that adding an electron to an atom actually requires energy. So if you go by the sign convention, the electron affinity of bromine is actually positive because energy is being released when you add an electron to bromine.Bromine is located below chlorine in group 17, which means that a bromine atom is larger than a chlorine atom. This of course implies that the outermost electrons are located further away from the nucleus in bromine’s case. In other words, chlorine has a higher electron affinity than bromine.
| Halogen | Electron Affinity (kJ/mol) |
|---|---|
| Chlorine | -349.0 |
| Bromine | -324.6 |
| Iodine | -295.2 |
| Astatine | -270.1 |
Why is the electron affinity of bromine negative?
When electron affinity is negative, it implies that adding an electron to an atom actually requires energy. So if you go by the sign convention, the electron affinity of bromine is actually positive because energy is being released when you add an electron to bromine.
Does Br have high electron affinity?
Bromine is located below chlorine in group 17, which means that a bromine atom is larger than a chlorine atom. This of course implies that the outermost electrons are located further away from the nucleus in bromine’s case. In other words, chlorine has a higher electron affinity than bromine.
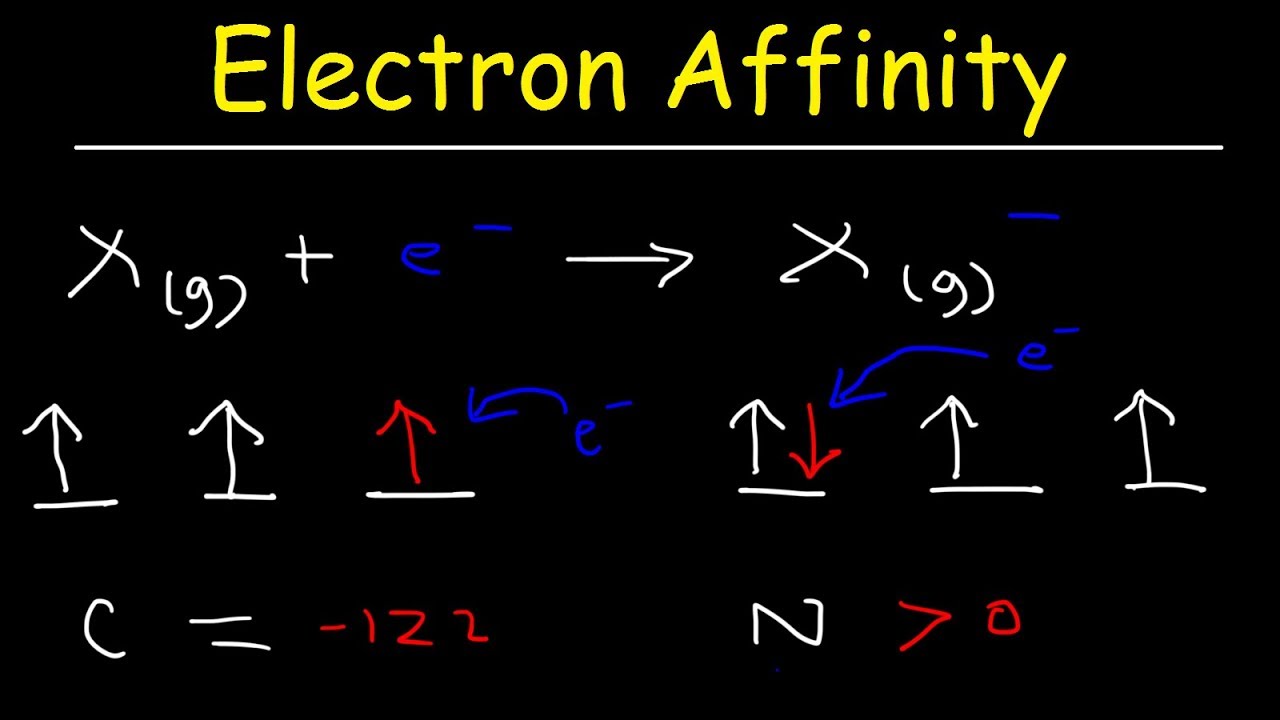
Electron Affinity Trend, Basic Introduction, Chemistry
Images related to the topicElectron Affinity Trend, Basic Introduction, Chemistry

Why does bromine have a high electron affinity?
Moreover, bromine has an extra full shell of core electrons between the nucleus and the outermost shell. These extra core electrons will shield the outermost electrons from the nucleus.
Does bromine or iodine have a higher electron affinity?
| Halogen | Electron Affinity (kJ/mol) |
|---|---|
| Chlorine | -349.0 |
| Bromine | -324.6 |
| Iodine | -295.2 |
| Astatine | -270.1 |
Is Br negative or positive?
A bromide ion is the negatively charged form (Br−) of the element bromine, a member of the halogens group on the periodic table. Most bromides are colorless.
How do you find electron affinity?
The amount of energy released when a neutral atom in its gaseous state accepts an electron and gets converted into a negatively charged ion is known as electron affinity. X ( g ) + e − → X − ( g ) + E . A . Therefore, the electron affinity of chlorine is – 349 KJ/mol.
Which has the greatest electron affinity SE Cl or Br?
The electron affinity of Cl is 3.612 eV. The electron affinity of Br is 3.363 eV. Hence, chlorine has the highest electron affinity than fluorine and bromine. Therefore, the correct option is b.
See some more details on the topic What is the electron affinity of bromine? here:
A7: Electron Affinities – Chemistry LibreTexts
ZElementNameElectron affinity (eV)Electron affinity (kJ/mol)12DDeuterium0.754 59(8)72.807(8)11HHydrogen0.754 195(19)72.769(2)2HeHelium‑19.7‑View 121 more rows
The electron affinity of bromine atom is equal to the … – Toppr
Electron affinity is nothing but adding of electron to an atom whereas IP is removal of electron. Hence, electron affinity of bromine is equal to the ionization …
[Q Solved] Frequently Asked Questions on Electron Affinity Of …
The numerical value of the electron affinity of Bromine is -325 kJ mol-1. According to the trend of electron affinity, the electron affinity …
The Electron Affinity of Bromine and a Study of Its …
The electron affinity of bromine has been measured by determining the ratio of ions to electrons leaving a hot tungsten surface in contact …
Which electron has highest affinity?
Which Element Has the Highest Electron Affinity? Chlorine has the highest electron affinity among the elements. Its high affinity can be attributed to its large atomic radius, or size. Because chlorine’s outermost orbital is 3p, its electrons have a large amount of space to share with an incoming electron.
What has the lowest electron affinity?
The correct answer is Argon. Argon has all filled orbitals as well as a filled valence shell. As a result, it doesn’t want to lose or gain any electrons. Hence, argon has the lowest electron affinity.
What is the first electron affinity?
The first electron affinity is the energy released when 1 mole of gaseous atoms each acquire an electron to form 1 mole of gaseous -1 ions. It is the energy released (per mole of X) when this change happens. First electron affinities have negative values.
What is electron affinity? | Chemistry | Extraclass.com
Images related to the topicWhat is electron affinity? | Chemistry | Extraclass.com

Why the electron affinity of Br Br has a greater magnitude than that of II?
Which of the following best helps to explain why the electron affinity of Br has a greater magnitude than that of I? The ionization energy of BrBr is higher than that of II. But in terms of electron affinity, BrBr has one less electron shell than II does.
What is the electron affinity of fluorine?
…
Elements.
| Z | 9 |
|---|---|
| Element | F |
| Name | Fluorine |
| Electron affinity (eV) | 3.401 189 8(24) |
| Electron affinity (kJ/mol) | 328.164 9(3) |
Why is bromine negatively charged?
Below is the Lewis dot structure for a neutral bromine atom, which has seven valence electrons. Below is the Lewis dot structure for a Br− ion, which has eight valence electrons. The extra valence electron gives it a negative charge.
What is charge of bromine?
…
Table of Common Element Charges.
| Number | Element | Charge |
|---|---|---|
| 35 | bromine | 1-, 1+, 5+ |
| 36 | krypton | 0 |
| 37 | rubidium | 1+ |
| 38 | strontium | 2+ |
Is bromine a neutral atom?
The neutral atom of bromine has 35 electrons because the number of electrons equals the number of protons.
What is electron affinity of an element?
The electron affinity of an element is a measure of that element’s tendency to act as an oxidizing agent (an electron acceptor) and is generally related to the nature of the chemical bonds the element forms with other elements.
What is the electron affinity of oxygen?
Electron Affinity of Oxygen is 141 kJ/mol.
What is electron affinity and electronegativity?
Electronegativity is defined as a chemical property which decides the propensity of an atom to attract an electron. In the year 1932, Linus Pauling proposed the concept of electronegativity. Electron affinity is defined as the amount of energy liberated when a molecule or neutral atom acquires an electron from outside.
Why is electron affinity of fluorine more than bromine?
Fluorine is in period two of the periodic table, meaning that it contains electrons in only the first two energy levels at ground state. It has a higher ionization energy than bromine due to the fact that the valence electrons of fluorine are attracted more strongly to its positively charged atomic nuclei.
The Periodic Table: Atomic Radius, Ionization Energy, and Electronegativity
Images related to the topicThe Periodic Table: Atomic Radius, Ionization Energy, and Electronegativity

Why iodine has a lower electron affinity than bromine?
On moving from chlorine to bromine to iodine, the electron affinity decreases (becomes less negative). This is because the increase in atomic size decrease the effective nuclear charge. Hence, the additional electron feels less attraction by the large atom. Was this answer helpful?
Which of the following has maximum Cl Cl Br I?
Br has larger atomic size than Cl because the atomic size increases from top to bottom in a group. From top to bottom in a group, the number of shells increases. So, the atomic size increases.
Related searches to What is the electron affinity of bromine?
- ionization energy of bromine
- why electron affinity is negative
- electron affinity of fluorine
- what is the reaction that corresponds to the electron affinity of bromine
- what does electron affinity do
- what is the reaction that corresponds to the electron affinity of bromine brbr
- what is the electron affinity of o
- what is the electron of bromine
- lowest electron affinity
- what is the electron affinity of bromine
- what is the reaction that corresponds to the electron affinity of bromine br
- electron affinity of bromine equation
- what is the sign of electron affinity
- electron affinity trend
- electron affinity of chlorine
- electron affinity of oxygen
- electron affinity of sodium
- which elements has the lowest electron affinity
Information related to the topic What is the electron affinity of bromine?
Here are the search results of the thread What is the electron affinity of bromine? from Bing. You can read more if you want.
You have just come across an article on the topic What is the electron affinity of bromine?. If you found this article useful, please share it. Thank you very much.