Are you looking for an answer to the topic “What is the electron affinity of fluorine?“? We answer all your questions at the website Ecurrencythailand.com in category: +15 Marketing Blog Post Ideas And Topics For You. You will find the answer right below.
Electron Affinity of Fluorine is 328 kJ/mol.Fluorine has highest electron affinity in the periodic table.Solution. The less electron affinity of fluorine is due to its smaller size. Adding an electron to the 2p orbital in fluorine leads to a greater repulsion than adding an electron to the larger 3p orbital of chlorine. Hence, fluorine has less electron affinity than chlorine.
Is fluorine high in electron affinity?
Fluorine has highest electron affinity in the periodic table.
Why does fluorine has less electron affinity?
Solution. The less electron affinity of fluorine is due to its smaller size. Adding an electron to the 2p orbital in fluorine leads to a greater repulsion than adding an electron to the larger 3p orbital of chlorine. Hence, fluorine has less electron affinity than chlorine.
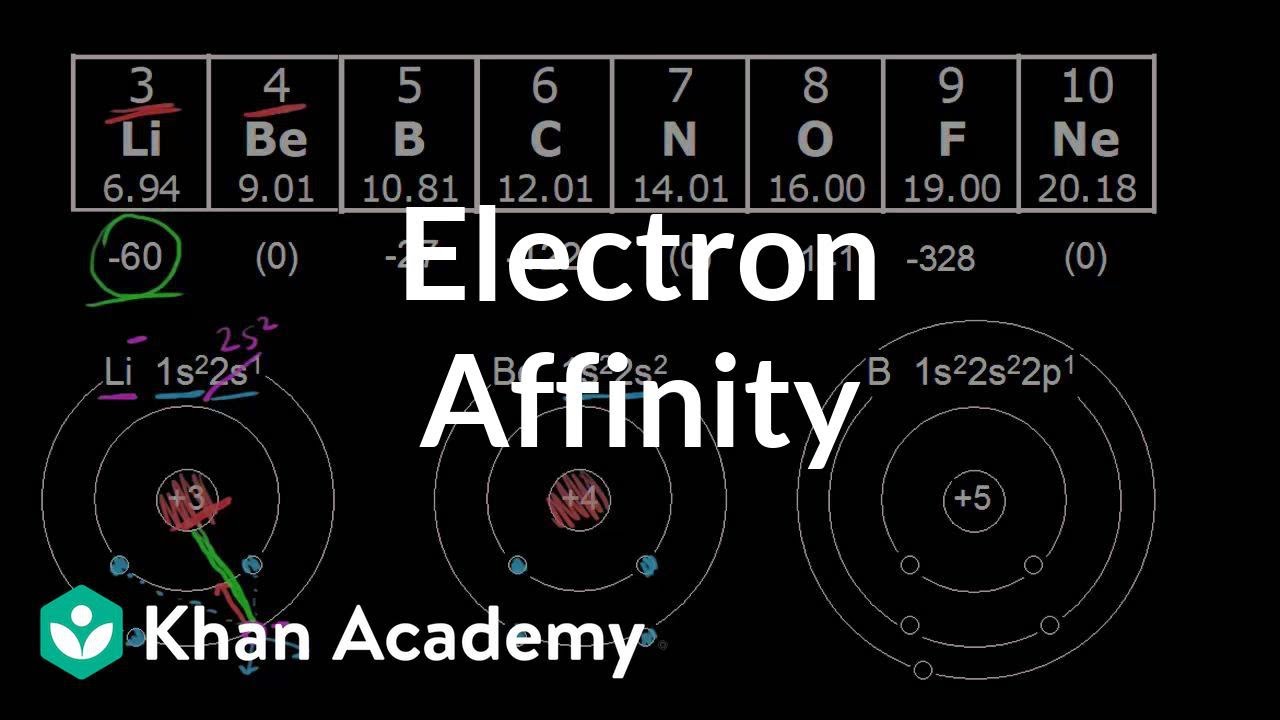
Electron affinity: period trend | Atomic structure and properties | AP Chemistry | Khan Academy
Images related to the topicElectron affinity: period trend | Atomic structure and properties | AP Chemistry | Khan Academy

Does fluorine have zero electron affinity?
Halogens have the highest electronegativity among elements and also electron affinity. Fluorine has the electronic configuration 1s22s22p5. As after gaining an electron it will attain a stable electronic configuration, it has a very high electron affinity.
Which has highest electron affinity?
Halogens has higher electron affinity and it is supposed to be for fluorine, but chlorine has higher electron affinity than fluorine due to fluorine’s smaller size. Hence, among given options chlorine has highest electron affinity.
How do you find electron affinity?
The amount of energy released when a neutral atom in its gaseous state accepts an electron and gets converted into a negatively charged ion is known as electron affinity. X ( g ) + e − → X − ( g ) + E . A . Therefore, the electron affinity of chlorine is – 349 KJ/mol.
Which has more electron affinity fluorine or chlorine?
Electronegativity of fluorine is greater than that of chlorine but electron affinity of chlorine is greater than that of fluorine.
What is the electronegativity of F?
See some more details on the topic What is the electron affinity of fluorine? here:
Electron Affinity – Chemistry LibreTexts
A fluorine atom has an electronic structure of 1s22s22px22py22pz1. It has 9 protons in the nucleus.The incoming electron …
Electron Affinity of Fluorine
Various theoretical and semi-empirical methods for determining electron affinities of atoms have been applied to fluorine. All results indicate a low value …
electron affinity – Chemguide
As you might have noticed, the first electron affinity of oxygen (-142 kJ mol-1) is less than that of fluorine (-328 kJ mol-1). Similarly sulphur’s (-200 kJ mol …
Electron affinity (data page) – Wikipedia
8 · O · Oxygen, 1.461 105(3), 140.975 2(3) ; 9, F · Fluorine, 3.401 189 8(24), 328.164 9(3).
Why is fluorine high electronegative?
Electronegativity of Fluorine
Fluorine is the most electronegative element because it has 5 electrons in it’s 2P shell. The optimal electron configuration of the 2P orbital contains 6 electrons, so since Fluorine is so close to ideal electron configuration, the electrons are held very tightly to the nucleus.
What is electron affinity in periodic table?
Electron affinity decreases down the groups and from right to left across the periods on the periodic table because the electrons are placed in a higher energy level far from the nucleus, thus a decrease from its pull.
What does 0 electron affinity mean?
Unlike ionization energies, which are always positive for a neutral atom because energy is required to remove an electron, electron affinities can be negative (energy is released when an electron is added), positive (energy must be added to the system to produce an anion), or zero (the process is energetically neutral) …
Fluorine has lower electron affinity than chlorine because of
Images related to the topicFluorine has lower electron affinity than chlorine because of

What has the lowest electron affinity?
The correct answer is Argon. Argon has all filled orbitals as well as a filled valence shell. As a result, it doesn’t want to lose or gain any electrons. Hence, argon has the lowest electron affinity.
Which has higher electron affinity fluorine or neon?
Thus, noble gases have the least electron affinity in a period. Hence, we can conclude that in a period, fluorine (halogen) has higher electron affinity than neon (noble gas).
Which halogen has the highest electron affinity?
The element with the highest electron affinity among halogens is Chlorine (Cl).
What is the electron affinity of bromine?
Electron Affinity of Bromine is 324.6 kJ/mol.
What is electron affinity and electronegativity?
Electronegativity is defined as a chemical property which decides the propensity of an atom to attract an electron. In the year 1932, Linus Pauling proposed the concept of electronegativity. Electron affinity is defined as the amount of energy liberated when a molecule or neutral atom acquires an electron from outside.
What is the electron affinity of oxygen?
…
Elements.
| Z | 8 |
|---|---|
| Element | 17O |
| Name | Oxygen |
| Electron affinity (eV) | 1.461 108 (4) |
| Electron affinity (kJ/mol) | 140.975 5(3) |
What is the electron affinity of carbon?
The electron affinity of carbon is 153.9 kJ mol‑1.
Which has greater electron affinity fluorine or bromine?
…
Electron Affinity (decreases down the group)
| Halogen | Electron Affinity (kJ/mol) |
|---|---|
| Bromine | -324.6 |
| Iodine | -295.2 |
| Astatine | -270.1 |
What is electron affinity? | Chemistry | Extraclass.com
Images related to the topicWhat is electron affinity? | Chemistry | Extraclass.com

Why does fluorine have a higher electron affinity than oxygen?
Increase in nuclear charge increases the effective force on the valence electron and hence the incoming electron can be held strongly and hence oxygen will have less electron affinity or fluorine will have greater electron affinity.
Why is the first electron affinity of O less than that of F?
As you might have noticed, the first electron affinity of oxygen (-142 kJ mol–1) is less than that of fluorine (-328 kJ mol–1). Similarly sulphur’s (-200 kJ mol–1) is less than chlorine’s (-349 kJ mol–1). Why? It’s simply that the Group 6 element has 1 less proton in the nucleus than its next door neighbour in Group 7.
Related searches to What is the electron affinity of fluorine?
- does fluorine have the highest electron affinity
- what is the reaction that corresponds to the electron affinity of fluorine f
- what is the reaction that corresponds to the electron affinity of fluorine
- why does fluorine have a high electron affinity
- electron affinity table
- what is the electron affinity of o
- lowest electron affinity
- what is the electron affinity of fluorine
- does fluorine have a high electron affinity
- why fluorine has less electron affinity
- electron affinity of li
- electron affinity value of fluorine
- nitrogen electron affinity
- why is the electron affinity of fluorine negative
- electron affinity trend
- electron affinity of chlorine
- electron affinity of oxygen
- electron affinity of sodium
Information related to the topic What is the electron affinity of fluorine?
Here are the search results of the thread What is the electron affinity of fluorine? from Bing. You can read more if you want.
You have just come across an article on the topic What is the electron affinity of fluorine?. If you found this article useful, please share it. Thank you very much.