Are you looking for an answer to the topic “What is the electron affinity of noble gases?“? We answer all your questions at the website Ecurrencythailand.com in category: +15 Marketing Blog Post Ideas And Topics For You. You will find the answer right below.
Electron affinity of noble gas elements is zero.Any electrons added to a noble gas would have to be the first electron in a new (larger) energy level. This causes the noble gases to have essentially zero electron affinity.Electron affinity of noble gas is near zero. Noble gas has completely filled valence shells and stable octets. It does not accept electrons easily as they have no deficiency, also adding electrons produces repulsion between the electrons.

Do noble gases have electron affinity?
Any electrons added to a noble gas would have to be the first electron in a new (larger) energy level. This causes the noble gases to have essentially zero electron affinity.
What is the electron affinity of the noble gases and why?
Electron affinity of noble gas is near zero. Noble gas has completely filled valence shells and stable octets. It does not accept electrons easily as they have no deficiency, also adding electrons produces repulsion between the electrons.
The electron affinity for the inert gases is
Images related to the topicThe electron affinity for the inert gases is

Why is electron affinity for noble gases low?
Noble gas elements have completely filled outer-shell. Such electronic configurations are highly stable and as such noble gases find it difficult to accept electrons. Thus electron affinity of noble gas elements is zero.
Do noble gases have highest electron affinity in a period?
Solution : All noble gases have stable configuration. Therefore, they can not take any electron means that they have no affinity for electrons. High electron affinity shows that electron is strongly bonded to the atom. Here both assertion and reason are false.
Which electron has highest affinity?
Which Element Has the Highest Electron Affinity? Chlorine has the highest electron affinity among the elements. Its high affinity can be attributed to its large atomic radius, or size. Because chlorine’s outermost orbital is 3p, its electrons have a large amount of space to share with an incoming electron.
Why electron affinity decreases down the group?
Electron affinity decreases down the groups and from right to left across the periods on the periodic table because the electrons are placed in a higher energy level far from the nucleus, thus a decrease from its pull.
What is the electron affinity of carbon?
The electron affinity of carbon is 153.9 kJ mol‑1.
See some more details on the topic What is the electron affinity of noble gases? here:
7.5: Electron Affinities – Chemistry LibreTexts
Elements that do not form stable ions, such as the noble gases, are assigned an effective electron affinity that is greater than or equal to …
Assertion Electron affinity of noble gas elements is class 11 …
Electron affinity of noble gas is near zero. Noble gas has completely filled valence shells and stable octets. It does not accept electrons easily as they have …
Electron affinity of noble gas elements is zero. Give … – Doubtnut
Electron affinity of noble gas elements is zero. Give reasons. · Noble gas elements have completely filled outer-shell. Such electronic configurations are highly …
High School Chemistry/Electron Affinity – Wikibooks
Lesson SummaryEdit · Electron affinity is the energy required (or released) when an electron is added to a gaseous atom or ion. Electron affinity generally …
What has the lowest electron affinity?
The correct answer is Argon. Argon has all filled orbitals as well as a filled valence shell. As a result, it doesn’t want to lose or gain any electrons. Hence, argon has the lowest electron affinity.
Why are electron affinities of noble gases zero? Arrange halogens in increasing order of electron
Images related to the topicWhy are electron affinities of noble gases zero? Arrange halogens in increasing order of electron

How do you find electron affinity?
The amount of energy released when a neutral atom in its gaseous state accepts an electron and gets converted into a negatively charged ion is known as electron affinity. X ( g ) + e − → X − ( g ) + E . A . Therefore, the electron affinity of chlorine is – 349 KJ/mol.
What is the electron affinity trend?
Electron affinity generally increases across a period in the periodic table and sometimes decreases down a group. These trends are not necessarily universal. The chemical rationale for changes in electron affinity across the periodic table is the increased effective nuclear charge across a period and up a group.
Why does electron affinity increase from bottom to top?
The general trends of the electron affinity are that it increases from left to right across the periodic table due to an increase in the nuclear charge, and it increases from bottom to top due to the effect of atomic size.
Why second electron affinity is positive?
The second electron affinity is a positive value as energy is needed to add a second electron to an ion with a pre-existing negative charge. This negative charge repels the incoming electron. Adding an electron is thus an endothermic process, and requires energy input.
Does electron affinity increase from top to bottom?
Electron affinity decreases from top to bottom within a group. This is caused by the increase in atomic radius.
What is the electron affinity of helium?
Electron affinity of helium (1s2s)3S
Extensive studies of patterns of convergence for the energy show that Enr=−2.178074(10), which together with Pekeris’ accurate Enr He(1s2s)3S yields a nonrelativistic electron affinity Anr=77.4(3) meV.
Electron Affinity Trend, Basic Introduction, Chemistry
Images related to the topicElectron Affinity Trend, Basic Introduction, Chemistry
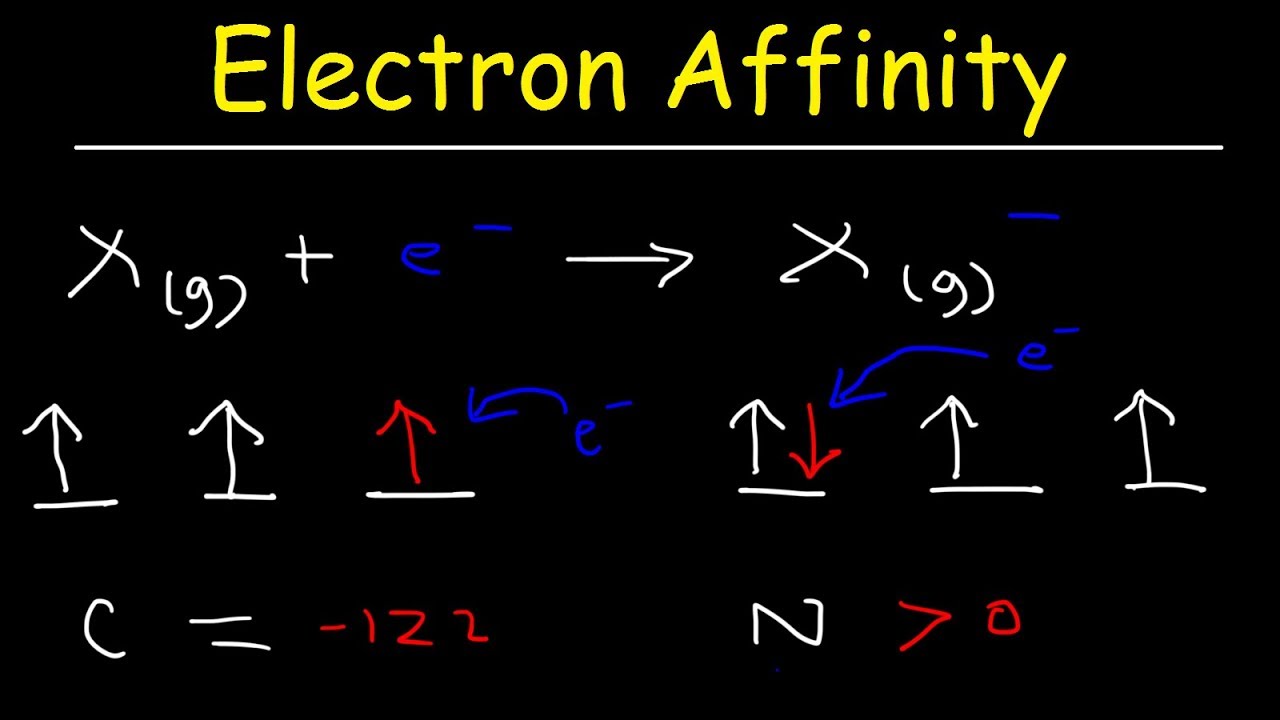
Which has more electron affinity O or O?
Actually, since O− is negatively-charged, it would be more difficult to accept an electron, and significant coulombic repulsion would make the electron affinity of O− more positive than that of O (which is negative).
What is the electron affinity of carbon and nitrogen?
The negative ions of boron and nitrogen are found to be unstable, the electron affinity being ca – 0.14 and – 0.72 ev, respectively. The electron affinities of carbon and oxygen atoms are 1.75 and 1.13 ev, respectively.
Related searches to What is the electron affinity of noble gases?
- do noble gases have ionization energy
- why noble gas have zero electron affinity
- do noble gases have electron affinity
- what is the electron affinity of noble gases
- electron affinity values of noble gases
- why do noble gases have weak electron affinity
- electron affinity exceptions
- ionization energy of noble gases
- highest electron affinity
- why electronegativity of noble gases is zero
- why do noble gases have low electron affinity
- electronegativity of noble gases
- electron affinity trend
- why electron affinity of noble gases is positive
- do noble gases have a high electron affinity
Information related to the topic What is the electron affinity of noble gases?
Here are the search results of the thread What is the electron affinity of noble gases? from Bing. You can read more if you want.
You have just come across an article on the topic What is the electron affinity of noble gases?. If you found this article useful, please share it. Thank you very much.