Are you looking for an answer to the topic “What is the electron affinity of selenium?“? We answer all your questions at the website Ecurrencythailand.com in category: +15 Marketing Blog Post Ideas And Topics For You. You will find the answer right below.
Electron Affinity of Selenium is 195 kJ/mol.The electron affinity of selenium is greater than oxygen. The reason for this is the small size of oxygen atom as compared to the selenium atom and so its charge density is high,and when then if we add an electron to oxygen atom then the repulsion between electrons will increase.But the size of Sulphur and selenium is not that different. Therefore, combining the effect of size and electron gain enthalpy the electron affinity of Sulphur is a bit higher than selenium and that of oxygen is least in the group. Hence, The correct order of electron affinity is $ O < Se < S $ .
Which has more electron affinity oxygen or selenium?
The electron affinity of selenium is greater than oxygen. The reason for this is the small size of oxygen atom as compared to the selenium atom and so its charge density is high,and when then if we add an electron to oxygen atom then the repulsion between electrons will increase.
Why selenium has less electron affinity than Sulenur?
But the size of Sulphur and selenium is not that different. Therefore, combining the effect of size and electron gain enthalpy the electron affinity of Sulphur is a bit higher than selenium and that of oxygen is least in the group. Hence, The correct order of electron affinity is $ O < Se < S $ .
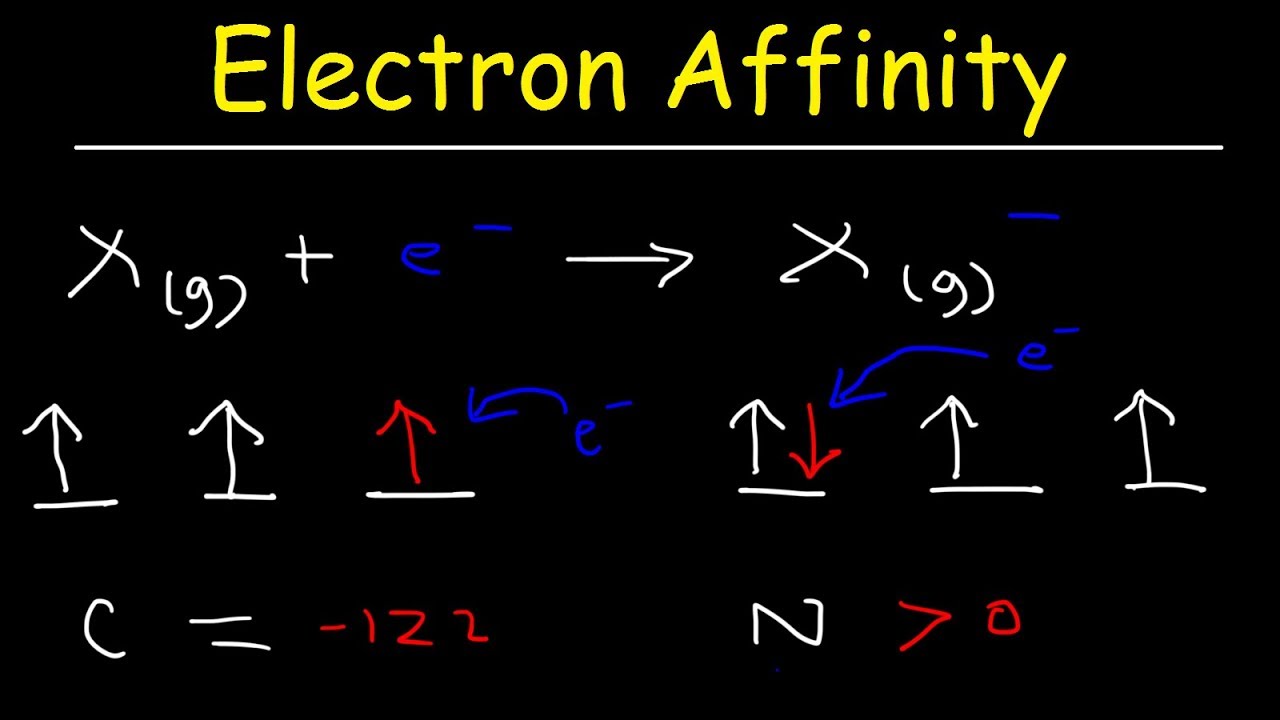
Electron Affinity Trend, Basic Introduction, Chemistry
Images related to the topicElectron Affinity Trend, Basic Introduction, Chemistry

Does selenium have a larger electron affinity than potassium?
So that is our answer to Part B. And you can also confirm because potassium is a metal and selenium is a nonmetal non metals tend to have higher electron affinities because they’re the ones that gain electrons as opposed to metals which lose electrons.
Which is correct order of electron affinity?
Electron affinity is the tendency is attract shared pair of electron towards itself. Its value decreases in a group and increases in a period. The correct order of electron affinities is as follows N<O<S<Cl.
Why electron affinity of Se is more than O?
Solution : `S` has high electron affinity because in oxygen there is ‘more interelectronic repulsion than sulphur, therefore, more energy is released in case of sulphur on gaining electrons .
What is electron affinity of an element?
The electron affinity of an element is a measure of that element’s tendency to act as an oxidizing agent (an electron acceptor) and is generally related to the nature of the chemical bonds the element forms with other elements.
Which electron has highest affinity?
Which Element Has the Highest Electron Affinity? Chlorine has the highest electron affinity among the elements. Its high affinity can be attributed to its large atomic radius, or size. Because chlorine’s outermost orbital is 3p, its electrons have a large amount of space to share with an incoming electron.
See some more details on the topic What is the electron affinity of selenium? here:
Selenium » properties of free atoms – WebElements Periodic …
Selenium atoms have 34 electrons and the shell structure is 2.8.18.6. The ground state electron configuration of ground state gaseous neutral selenium is [Ar].
️ Electron Affinity of Selenium (Se) [& Color, Uses, Discovery
In chemistry, the electron affinity of an atom of Selenium is defined as the amount of energy released or spent when an electron is added to a neutral atom …
Why is the electron affinity of Selenium greater than Oxygen?
The electron affinity of selenium is greater than oxygen. The reason for this is the small size of oxygen atom as compared to the selenium atom and so its …
Electron affinity of selenium measured by photodetachment …
The electron affinity eA(Se) of selenium is measured by photodetachment microscopy on a cesium-sputtering-produced Se− ion beam using a …
What is electron affinity equation?
The electron affinity (Eea) of an atom or molecule is defined as the amount of energy released when an electron is attached to a neutral atom or molecule in the gaseous state to form an anion. X(g) + e− → X−(g) + energy.
Why does group 16 have low electron affinity?
This is prominent in oxygen and fluorine. Because of the small size, all electrons are packed in a small volume and thus, an incoming electron faces a lot of repulsion. This lowers the stability of the species formed and hence leads to lower electron affinity.
Which element has a higher electron affinity Se Te?
The overall order is Se < Te < Sb, so Se has the most negative electron affinity among the three elements.
What is the order of electron affinity in Group 16?
Moving down across a group 16, we have, O, S, Se, and then Te. We know that as we move down a group, the electron gain enthalpy decreases so the trend should be O > S > Se > Te.
What is electron affinity? | Chemistry | Extraclass.com
Images related to the topicWhat is electron affinity? | Chemistry | Extraclass.com

What has the lowest electron affinity?
The correct answer is Argon. Argon has all filled orbitals as well as a filled valence shell. As a result, it doesn’t want to lose or gain any electrons. Hence, argon has the lowest electron affinity.
What is positive electron affinity?
The electron affinity is the energy change when an atom gains electrons. The convention is that the higher or more positive the electron affinity value, the more readily the atom accepts an electron.
How do you find the lowest electron affinity?
The less valence electrons an atom has, the least likely it will gain electrons. Electron affinity decreases down the groups and from right to left across the periods on the periodic table because the electrons are placed in a higher energy level far from the nucleus, thus a decrease from its pull.
Which is correct for F & Cl?
F > Cl > Cl- > F.
Why does electron affinity decrease down group 7?
When moving down a group, the electron affinity generally decreases. This is because as you go down the period table, new valence shells are added increasing the atomic radius. The new orbital is further away from the nucleus, meaning the attraction between the positively charged nucleus and the new electron decrease.
Which one N or O has more electron affinity and why?
Answer: Oxygen has more electron affinity because Nitrogen gains more stability by attaining partial configuration.
Which has more Ege O or S?
S has high electron gain enthalpy because in sulphur there is more inter-electronic repulsion than oxygen, therefore, more energy is released in case of sulphur on gaining electrons.
What is the electronegativity of Se?
What is the electron affinity of magnesium?
The electron affinity of magnesium is 0 kJ mol‑1.
What is Electron Affinity?
Images related to the topicWhat is Electron Affinity?

What is electron affinity class 11?
Electron affinity is defined as the amount of energy released when an electron is added to a neutral atom to form an anion. It is the change in the potential energy of an atom when an electron is added to a neutral gaseous atom to form a negative ion. The unit of electron affinity is KJ per mole (KJmol).
What is electron affinity and electronegativity?
Electronegativity is defined as a chemical property which decides the propensity of an atom to attract an electron. In the year 1932, Linus Pauling proposed the concept of electronegativity. Electron affinity is defined as the amount of energy liberated when a molecule or neutral atom acquires an electron from outside.
Related searches to What is the electron affinity of selenium?
- potassium electron affinity
- what is the electron affinity of selenium and zinc
- what is the electron affinity of selenium in the body
- what is the electron affinity of selenium 4
- electron affinity of sulphur
- electron affinity of bromine
- electron affinity of tellurium
- electron affinity of o
- electron affinity of polonium
- what is the electron affinity of selenium 3
- electron affinity of as
- electron affinity of chlorine
Information related to the topic What is the electron affinity of selenium?
Here are the search results of the thread What is the electron affinity of selenium? from Bing. You can read more if you want.
You have just come across an article on the topic What is the electron affinity of selenium?. If you found this article useful, please share it. Thank you very much.