Are you looking for an answer to the topic “What is the electron configuration of the Mg2+ cation?“? We answer all your questions at the website Ecurrencythailand.com in category: +15 Marketing Blog Post Ideas And Topics For You. You will find the answer right below.
Mg2+ has an electronic configuration of 1s² 2s² 2p^6, i.e., has a total number of 10 electrons similar to that of a Noble gas [Ne] instead of Mg 12 as its total number of electrons and configuration of 1s² 2s² 2p6 3s².for Mn2+ i would say 1s2 2s2 2p6 3s2 3p6 4s0 3d5 because the electrons are always removed from the 4s orbital first.Magnesium(2+) is a magnesium cation, a divalent metal cation and a monoatomic dication.

What is the electron configuration of a Mn2+ cation?
for Mn2+ i would say 1s2 2s2 2p6 3s2 3p6 4s0 3d5 because the electrons are always removed from the 4s orbital first.
Is Mg2+ a cation or anion?
Magnesium(2+) is a magnesium cation, a divalent metal cation and a monoatomic dication.
Electron Configuration of Ions – Mg2+, P3-, Fe2+, Fe3+
Images related to the topicElectron Configuration of Ions – Mg2+, P3-, Fe2+, Fe3+

What is the electron configuration of mg3+?
A neutral manganese atom also has 25 electrons. However, the manganese 3+ ion, Mn3+ , has 22 electrons. This gives it 3 more protons than electrons, which gives it the 3+ charge. The electron configuration in noble gas shorthand for a neutral Mn atom is [Ar]3d54s2 .
Which element has the electron configuration 1s²2s²2p⁶3s²3p⁶4s²3d¹⁰4p²?
Germanium has the electron configuration 1s²2s²2p⁶3s²3p⁶4s²3d¹⁰4p².
Which electrons are lost to form the Mn2+ cation?
This means that a neutral manganese atom must have 25 electrons surrounding its nucleus. Consequently, the manganese(II) cation, Mn2+ , which is formed when a neutral manganese atom loses 2 electrons, will have a total of 23 electrons surrounding its nucleus.
How many electrons does Mg2+ have?
Answer and Explanation: There are 10 electrons in a Mg2+ ion. A neutral atom of magnesium would have 12 electrons to balance out the positive charge of the 12…
What is Mg2+ in chemistry?
Chemical Information. FooDB Name. Mg2+ Description. Magnesium, also known as magnesium ion or magnesium, ion (mg(2+)), is a member of the class of compounds known as homogeneous alkaline earth metal compounds.
See some more details on the topic What is the electron configuration of the Mg2+ cation? here:
Electron Configuration for Magnesium (Mg) – TerpConnect
We’ll put six in the 2p orbital and then put the remaining two electrons in the 3s. Therefore the Magnesium electron configuration will be 1s22s22p63s2.
Magnesium(Mg) electron configuration and orbital diagram
The ground state electron configuration of magnesium is 1s2 2s2 2p6 3s2. After the electron configuration, the last shell of the magnesium atom has two …
What is the noble gas configuration for Mg 2?
What’s the electron configuration for mg2+? … Therefore the Magnesium electron configuration will be 1s22s22p63s2. The configuration notation …
How is Mg2+ formed?
Magnesium is in Group 2. It has two electrons in its outer shell. When these electrons are lost, a magnesium ion, Mg 2+, is formed.
Which element has the electron configuration 1s22s22p63s23p5?
The electron configuration for chlorine is 1s2 2s2 2p6 3s2 3p5.
How many protons does Mg2+ have?
So, Mg2+ has 12 protons, 12 neutrons and 10 electrons.
How do you find the electron configuration?
To calculate an electron configuration, divide the periodic table into sections to represent the atomic orbitals, the regions where electrons are contained. Groups one and two are the s-block, three through 12 represent the d-block, 13 to 18 are the p-block and the two rows at the bottom are the f-block.
Mg 2+ Electron Configuration (Magnesium Ion)
Images related to the topicMg 2+ Electron Configuration (Magnesium Ion)

Which is bigger Mg or Mg2+?
Answer. So Mg atom will be larger as it has more electrons than Mg 2+ and thus will have larger atomic radii.
What is the electronic configuration of magnesium 24 12 Mg?
2,6,4.
How many electrons does Mg have?
The most common and stable type of magnesium atom found in nature has 12 protons, 12 neutrons, and 12 electrons (which have a negative charge).
What element is 1s²2s²2p⁶?
Answer. Answer: The electron configuration for Copper (Cu) is: 1s2 2s2 2p6 3s3 3p6 4s2 3d7.
Which of the following is the electron configuration for MN?
The electron configuration of manganese, atomic number 25, is 1s2222p63s23p63d54s2 .
Which of these elements has 5 valence electrons?
Explanation: The elements of group 15 (column) VA of the periodic table all have electron configurations of s2p3, giving them five valence electrons. These elements include Nitrogen (N), Phosphorus (P), Arsenic (As), Antimony (Sb) and Bismuth (Bi).
What atom goes with this electron configuration 1s2 2s2 2p3?
1. Element with electron configuration 1s2 2s2 2p3 is Nitrogen (N.)
How many unpaired electrons does Mn 2 plus have?
Hence, there are 5 unpaired electrons in Mn2+
What is the name of the ion Mg2+?
Magnesium ions (Mg2+) are the most abundant divalent metal ions within cells.
Electron Configuration – Basic introduction
Images related to the topicElectron Configuration – Basic introduction
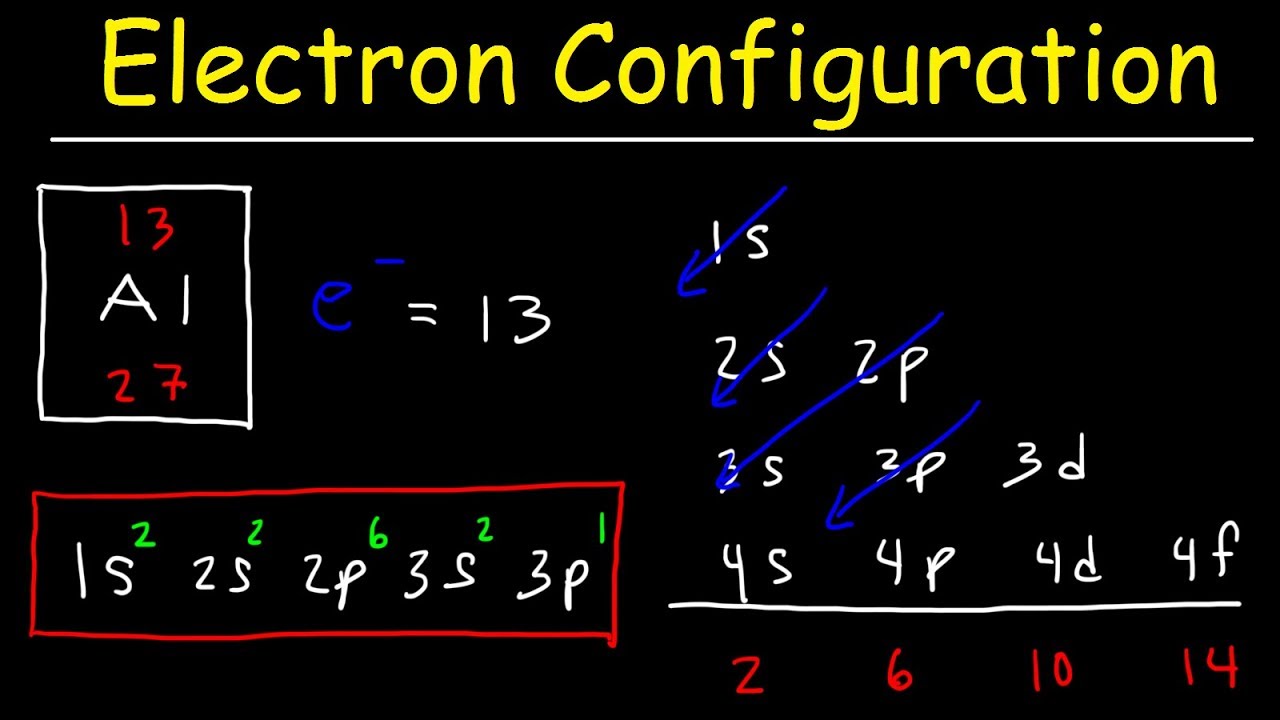
What is the atomic number of Mg2+?
atomic number of Mg=12 but Mg2+
How many valence electrons does Mg2+ have?
So if you add them up, then subtract two to account for the 2+ ionic charge, you will get the right number of electrons. In either case, you will end up with 12−2=10 electrons.
Related searches to What is the electron configuration of the Mg2+ cation?
- what is the electron configuration of mg2+
- magnesium electron configuration long form
- electron configuration of aluminum 13
- what is the electronic configuration of magnesium ion
- what is the electron configuration of the mg2 cation and anion
- electron configuration of sodium
- electron configuration of f
- electron configuration of iron
- mg2+ valence electrons
- mg2 valence electrons
- what is the electron configuration of the mg2 cation ion
- electron configuration of f-
- give the ground state electron configuration for mg2
Information related to the topic What is the electron configuration of the Mg2+ cation?
Here are the search results of the thread What is the electron configuration of the Mg2+ cation? from Bing. You can read more if you want.
You have just come across an article on the topic What is the electron configuration of the Mg2+ cation?. If you found this article useful, please share it. Thank you very much.