Are you looking for an answer to the topic “What is the electron shielding effect?“? We answer all your questions at the website Ecurrencythailand.com in category: +15 Marketing Blog Post Ideas And Topics For You. You will find the answer right below.
The shielding effect can be defined as a reduction in the effective nuclear charge on the electron cloud, due to a difference in the attraction forces on the electrons in the atom. It is a special case of electric-field screening. This effect also has some significance in many projects in material sciences.Ans: The inner electrons shield the outer electrons from the nuclear force thereby reducing the nuclear hold on the outermost electrons, this effect within atom is called shielding effect. For example, in sodium the electrons in first and second shells shield the electron in the third shell from the nuclear force.Electron shielding refers to the blocking of valence shell electron attraction by the nucleus, due to the presence of inner-shell electrons. Electrons in an s orbital can shield p electrons at the same energy level because of the spherical shape of the s orbital.
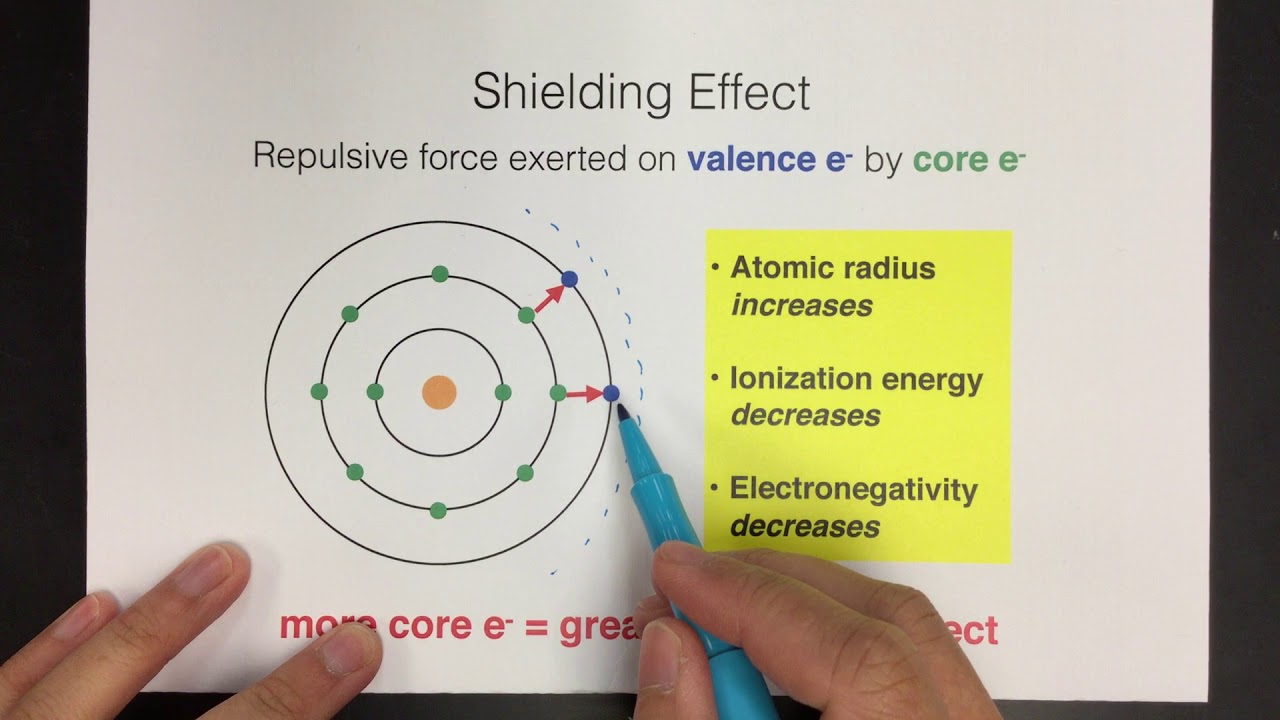
What is shielding effect with example?
Ans: The inner electrons shield the outer electrons from the nuclear force thereby reducing the nuclear hold on the outermost electrons, this effect within atom is called shielding effect. For example, in sodium the electrons in first and second shells shield the electron in the third shell from the nuclear force.
What is electron shielding in simple words?
Electron shielding refers to the blocking of valence shell electron attraction by the nucleus, due to the presence of inner-shell electrons. Electrons in an s orbital can shield p electrons at the same energy level because of the spherical shape of the s orbital.
Shielding Effect
Images related to the topicShielding Effect

What is the shielding effect and what causes it?
Shielding is caused by the combination of partial neutralization of nuclear charge by core electrons, and by electron-electron repulsion. The amount of charge felt by an electron depends on its distance from the nucleus.
What is shielding effect short answer?
When the number of inner electrons is greater, they shield the outermost electron from the nucleus so that the outermost electron becomes free from any nuclear attraction. This is called the shielding or screening effect.
What is shielding effect class 11th?
Hint: Shielding effect is the phenomenon of the atomic world where the valence electrons are shielded from the attraction towards the nucleus of an atom. This can be seen more in 3d elements.
What is shielding effect class 10th?
The screening effect is also known as the shielding effect. The phenomenon occurs when the nucleus reduces its force of attraction on the valence electrons due to the presence of electrons in the inner shell. This is known as a screening effect.
What is electron shielding quizlet?
Electron Shielding. When the inner electrons shield the outer electrons from the full attractive force of the nucleus. Valence Electrons. Outer most electrons responsible for chemical reactivity/chemical properties.
See some more details on the topic What is the electron shielding effect? here:
6.18: Electron Shielding – Chemistry LibreTexts
Electron shielding refers to the blocking of valence shell electron attraction by the nucleus, due to the presence of inner-shell electrons.
Electron Shielding: Definition and Examples – Chemistry Learner
In other words, the inner electrons shield the outer electrons from the nucleus. This phenomenon is known as electron shielding. The higher the number of …
What is shielding effect and screening effect? – BYJU’S
When the number of inner electrons is greater, they shield the outermost electron from the nucleus so that the outermost electron becomes free from any …
Effective Nuclear Charge – Electronic Structure – MCAT Content
Electrons in an atom can shield each other from the pull of the nucleus. This effect, called the shielding effect, describes the decrease in the attraction …
Does shielding increase down a group?
1 Answer. Shielding increases DOWN a Group because the nuclear core is farther removed from the valence electrons.
Shielding | Properties of Matter | Chemistry | FuseSchool
Images related to the topicShielding | Properties of Matter | Chemistry | FuseSchool

What is electron shielding How does it affect the trends?
The more shielding electrons you have, the lower the ENC, so the less force there is holding onto the outer shell electrons. If there is less force holding onto valence electrons, then they will be lost more easily, and likewise not gained as easily.
What is cause of shielding effect in elements?
Electrons in an atom can shield each other from the pull of the nucleus. This effect, called the shielding effect, describes the decrease in attraction between an electron and the nucleus in any atom with more than one electron shell.
What effect does electron shielding have on atomic radius?
Therefore, the more shielding that occurs, the less attraction there is between the outer electrons and nucleus, so the the further the electrons in the outer shell can spread out. This means the atomic radius will be larger.
How does electron shielding effect ionization energy?
The more electrons shielding the outer electron shell from the nucleus, the less energy required to expel an electron from said atom. The higher the shielding effect the lower the ionization energy (see diagram 2).
What is shielding effect class 12?
The decrease in the force of attraction exerted by the nucleus on the valence electrons due to the presence of electrons in the inner shells is called screening effect or shielding effect.
What is shielding effect Byjus?
In chemistry, the shielding effect is a state where an electron shields itself from the nucleus. The electrons present in the atom does not get affected by the nucleus. It is also called the screening effect.
What is shielding effect trends in periodic table?
When moving to the right of a period, the number of electrons increases and the strength of shielding increases. As a result, it is easier for valence shell electrons to ionize, and thus the ionization energy decreases down a group. Electron shielding is also known as screening. Trends.
What is electron shielding in atoms
Images related to the topicWhat is electron shielding in atoms

Why is the ionization energy of oxygen lower than nitrogen?
Oxygen also has an unexpectedly low ionisation energy, less than that of nitrogen. This is due to an electron being added to an already half full orbital in oxygen, which results in electron electron repulsion, which will lower the ionisation energy.
What is the 3p electron in an aluminum atom shielded by?
The electron being removed from an Al atom is a 3p electron, which is shielded by the two 3s electrons as well as all the inner core electrons.
Related searches to What is the electron shielding effect?
- how does electron shielding work
- does electron shielding increase across a period
- what is shielding in chemistry
- electron shielding example
- electron shielding and ionization energy
- shielding effect example
- what is shielding effect class 11
- what is a shielding electron
- what causes electron shielding
- shielding effect s p d f
- electron shielding trend
- what is the electron shielding effect
Information related to the topic What is the electron shielding effect?
Here are the search results of the thread What is the electron shielding effect? from Bing. You can read more if you want.
You have just come across an article on the topic What is the electron shielding effect?. If you found this article useful, please share it. Thank you very much.