Are you looking for an answer to the topic “What is the electronegativity difference between MG and BR?“? We answer all your questions at the website Ecurrencythailand.com in category: +15 Marketing Blog Post Ideas And Topics For You. You will find the answer right below.
The electronegativity difference between magnesium and bromine is 1,6. The bonds are polar. The molecule is symmetrical and is non-polar.For example, magnesium and bromine form an ionic bond. Magnesium has an oxidation number of +2 and bromine has an oxidation number of -1. The following shows an electron dot formula for the ionic compound that is formed by magnesium and bromine.Subtract the smaller electronegativity from the larger one to find the difference. For example, if we’re looking at the molecule HF, we would subtract the electronegativity of hydrogen (2.1) from fluorine (4.0). 4.0 – 2.1 = 1.9.
| H 2.1 | ||
|---|---|---|
| Li 1.0 | Be 1.5 | F 4.0 |
| Na 1.0 | Mg 1.2 | Cl 3.0 |
| K 0.9 | Ca 1.0 | Br 2.8 |
| Rb 0.9 | Sr 1.0 | I 2.5 |

Is Br and MG polar or nonpolar?
The electronegativity difference between magnesium and bromine is 1,6. The bonds are polar. The molecule is symmetrical and is non-polar.
What’s the electronegativity of MG?
Electronegativity, Basic Introduction, Periodic Trends – Which Element Is More Electronegative?
Images related to the topicElectronegativity, Basic Introduction, Periodic Trends – Which Element Is More Electronegative?

What bond occurs between MG Br?
For example, magnesium and bromine form an ionic bond. Magnesium has an oxidation number of +2 and bromine has an oxidation number of -1. The following shows an electron dot formula for the ionic compound that is formed by magnesium and bromine.
How do you find electronegativity difference?
Subtract the smaller electronegativity from the larger one to find the difference. For example, if we’re looking at the molecule HF, we would subtract the electronegativity of hydrogen (2.1) from fluorine (4.0). 4.0 – 2.1 = 1.9.
Does bromine and magnesium form ionic compounds?
For example, magnesium and bromine form an ionic bond. Magnesium has an oxidation number of +2 and bromine has an oxidation number of -1.
Why does Mg have a positive electron affinity?
additional energy is required for addition of electron to outermost shell of Mg i.e., this process is endothermic and thus the valueof electron affinity of Mg is positive.
How is the bond in Br2 different from the bond in MgF2?
The bond in Br2 is covalent while the bond in MgF2 is ionic.
See some more details on the topic What is the electronegativity difference between MG and BR? here:
Chemical Bonding Flashcards | Quizlet
Find the electronegativity difference between Mg and Br. 1.6. Image: Find the electronegativity … Image: Classify the bond that occurs between Mg and Br.
Bond Polarity of MgBr – ChemicalAid
Polar Covalent · 1.3 · 3.0 · |1.3 – 3.0| = 1.7 …
How to draw MgBr2 Lewis Structure? – Science Education and …
Magnesium has an electronegativity of 1.31, while bromine has an electronegativity of 2.96 in the MgBr2 molecule. The difference in electronegativity can be …
Periodic Trends — Electronegativity
When the electronegativity difference is between 0 and 2, the more electronegative element attracts the shared more strongly, but not strongly enough to remove …
Is bromine more electronegative than oxygen?
You will have to look up the Pauling scale yourself, but the O atom, first row, and rightmost on the table, will be most electronegative, followed by nitrogen and bromine.
What happens when magnesium and bromine react?
3 Magnesium reacts with bromine to form magnesium bromide.
Is magnesium bromide a covalent?
Answer and Explanation: Magnesium bromide is an ionic compound that is acidic in nature.
Electronegativity: bond character/bond type: electronegativity difference and predicting bond type
Images related to the topicElectronegativity: bond character/bond type: electronegativity difference and predicting bond type
How is magnesium bromide formed?
What Is The Formula For Magnesium Bromide? Treating hydrobromic acid (HBr) with magnesium oxide (MgO) and crystallizing the product. It can also be synthesized by reacting magnesium carbonate (MgCO3) and hydrobromic acids (HBr).
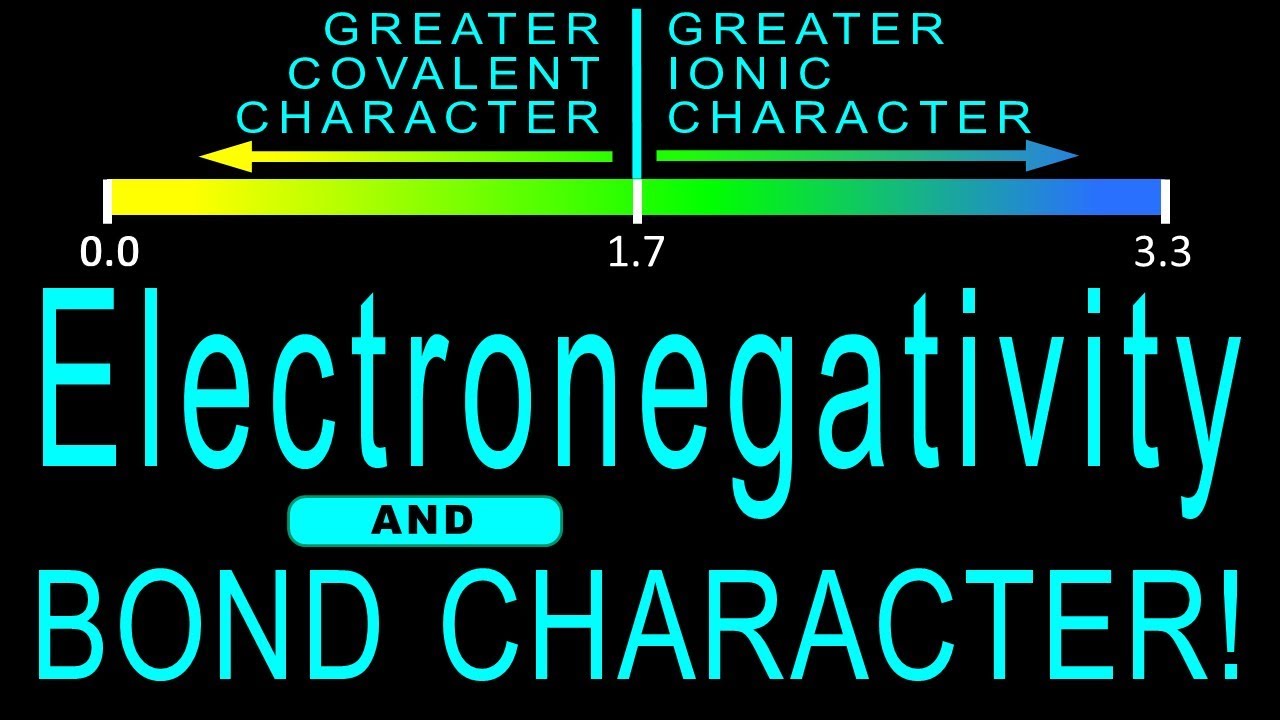
Is 0.4 electronegativity polar or nonpolar?
…
| ΔEN | Bonding | Bond Example |
|---|---|---|
| 0.0 – 0.4 | Nonpolar covalent bond | H-C, C-C |
| 0.5 – 0.9 | Slightly polar covalent bond | H-N, H-Cl |
| 1.0 – 1.3 | Moderately polar covalent bond | C-O, S-O |
What does the difference in electronegativity mean?
Electronegativity describes the degree to which an atom attracts electrons in a chemical bond. The difference in the electronegativity of two atoms determines their bond type. If the electronegativity difference is more than 1.7, the bond will have an ionic character.
How do you find the electronegativity difference between three atoms?
Note the electronegativity of the first and second elements. How to find electronegativity? Just use a periodic table which includes it and read it for the selected element. Subtract the two electronegativity values and you will have the electronegativity difference of the two elements or atoms.
Is mg a metal or nonmetal?
magnesium (Mg), chemical element, one of the alkaline-earth metals of Group 2 (IIa) of the periodic table, and the lightest structural metal. Its compounds are widely used in construction and medicine, and magnesium is one of the elements essential to all cellular life.
Is Mg a cation or anion?
Magnesium(2+) is a magnesium cation, a divalent metal cation and a monoatomic dication.
How many electrons does bromine gain or lose?
The atomic number for bromine is 35, which means it has 35 protons in its atomic nuclei. A neutral bromine atom would also have 35 electrons. In order for a bromine atom to become a 1− bromide ion, it would have to gain an additional electron.
What is the electron affinity of Br?
| Z | Element | Electron affinity (kJ/mol) |
|---|---|---|
| 35 | Br | 324.5371(3) |
| 36 | Kr | – |
| 37 | Rb | 46.884(2) |
| 38 | Sr | 5.023(6) |
How to get the Electronegativity difference of a compound
Images related to the topicHow to get the Electronegativity difference of a compound

What is the electronegativity of Mg and what type of element is it *?
The first scale of electronegativity was developed by Linus Pauling and on his scale magnesium has a value of 1.31 on a scale running from from about 0.7 (an estimate for francium) to 2.20 (for hydrogen) to 3.98 (fluorine).
Why does Mg not have an electron affinity?
The general trend is that electron affinity increases from left to right in the Periodic Table. Be and Mg are the two Group 2 elements closest to the head of the red arrow. Unlike most elements, they have Eea≤0 .
Related searches to What is the electronegativity difference between MG and BR?
- electronegativity of mg
- electronegativity of br
- electronegativity of f
- find the electronegativity difference between al and f
- what is the electronegativity difference between na and f
- electronegativity difference chart
- least electronegative element
- electronegativity of chlorine
- find the electronegativity difference between br and br
- what is the electronegativity difference of na and br
- what is the electronegativity difference of hf
- hydrogen electronegativity
- what is the electronegativity difference between mg and br
Information related to the topic What is the electronegativity difference between MG and BR?
Here are the search results of the thread What is the electronegativity difference between MG and BR? from Bing. You can read more if you want.
You have just come across an article on the topic What is the electronegativity difference between MG and BR?. If you found this article useful, please share it. Thank you very much.