Are you looking for an answer to the topic “What is the electronegativity difference of Na and Br?“? We answer all your questions at the website Ecurrencythailand.com in category: +15 Marketing Blog Post Ideas And Topics For You. You will find the answer right below.
Sodium bromide is an ionically bonded compound. The electronegativity of bromine is high enough and that the electromagnetic force between the Br and the Na atoms is great enough that an electron is transferred from the Na atom to the Br atom.The first scale of electronegativity was developed by Linus Pauling and on his scale bromine has a value of 2.96 on a scale running from from about 0.7 (an estimate for francium) to 2.20 (for hydrogen) to 3.98 (fluorine).
| Group | ||
|---|---|---|
| 3 | Na 0.93 | Cl 3.16 |
| 4 | K 0.82 | Br 2.96 |
| 5 | Rb 0.82 | I 2.66 |
| 6 | Cs 0.79 | At 2.2 |

What type of bond is Na and Br?
Sodium bromide is an ionically bonded compound. The electronegativity of bromine is high enough and that the electromagnetic force between the Br and the Na atoms is great enough that an electron is transferred from the Na atom to the Br atom.
What is the electronegativity of Br?
The first scale of electronegativity was developed by Linus Pauling and on his scale bromine has a value of 2.96 on a scale running from from about 0.7 (an estimate for francium) to 2.20 (for hydrogen) to 3.98 (fluorine).
How to get the Electronegativity difference of a compound
Images related to the topicHow to get the Electronegativity difference of a compound

What is the polarity of sodium and bromine?
Ionically bonded sodium bromide is a chemical compound. Bromine has a high electronegativity, which means that the electromagnetic force between the Br and Na atoms is strong enough to transfer one electron from the Na to the Br atom. Bromine is negatively charged as a result, whereas sodium is positively charged.
What is the electronegativity of nacl?
An ionic bond is formed when the electronegativity difference between atoms is greater than 1.7. In the case of sodium chloride, sodium has an electronegativity of 0.9 and chlorine has an electronegativity of 3.0.
What is the electronegativity of Na?
For example, sodium has an electronegativity of 0.93 and chlorine has an electronegativity of 3.16, so when sodium and chlorine form an ionic bond, in which the chlorine takes an electron away from sodium, forming the sodium cation, Na+, and the chloride anion, Cl–.
How do you find electronegativity difference?
Note the electronegativity of the first and second elements. How to find electronegativity? Just use a periodic table which includes it and read it for the selected element. Subtract the two electronegativity values and you will have the electronegativity difference of the two elements or atoms.
Why is Br more electronegative?
…
Pauling Electronegativity Scale.
| H 2.1 | K 0.9 |
|---|---|
| Ge 1.9 | |
| As 2.1 | |
| Se 2.4 | |
| Br 2.8 |
See some more details on the topic What is the electronegativity difference of Na and Br? here:
Difference between electronegativity values of sodium and …
The difference electronegativity values of sodium and bromine are; Sodium(Na) 0.9, Bromine(Br) 2.8 thus a difference of 1.9. What is the …
Sodium Bromide (BrNa) Bond Polarity – ChemicalAid
Bond Type, Ionic (Non-Covalent). Electronegativity (Na), 0.9. Electronegativity (Br), 3.0. Electronegativity Difference, |0.9 – 3.0| = 2.1.
electronegativity difference Na and Br – Brainly.ph
Answer: If there is a greater difference in electronegativity between atoms, the molecule has more ionic character.
Periodic Trends — Electronegativity
When atoms with an electronegativity difference of less than two units are joined together, the bond that is formed is a covalent bond, in which the electrons …
Electronegativity, Basic Introduction, Periodic Trends – Which Element Is More Electronegative?
Images related to the topicElectronegativity, Basic Introduction, Periodic Trends – Which Element Is More Electronegative?

How does electronegativity determine bond?
Electronegativity describes the degree to which an atom attracts electrons in a chemical bond. The difference in the electronegativity of two atoms determines their bond type. If the electronegativity difference is more than 1.7, the bond will have an ionic character.
Which of the following electronegativity differences would result in a polar covalent bond?
Polar Covalent Bonds
A bond in which the electronegativity difference between the atoms is between 0.4 and 1.7 is called a polar covalent bond.
Is Na+ ion polar or nonpolar?
Sodium Chloride (NaCl) which is an ionic compound acts as a polar molecule. Usually, the large difference in electronegativities in sodium and chlorine makes their bond polar.
Is Br2 nonpolar or polar?
Br2 (bromine) is nonpolar because, in this molecule, both bromine atoms have equal electronegativity, which causes both atoms to have the same charge distribution and results in a net-zero dipole moment. It is linear in structure.
Is B or Cl more electronegative?
…
Carbon is More Electronegative Than You Think.
| Element | Electronegativity (Pauling) |
|---|---|
| H | 2.2 [2.20] |
| P | 2.2 [2.19] |
| B | 2.0 [2.04] |
| Si | 1.9 [1.90] |
What is the electronegativity of B?
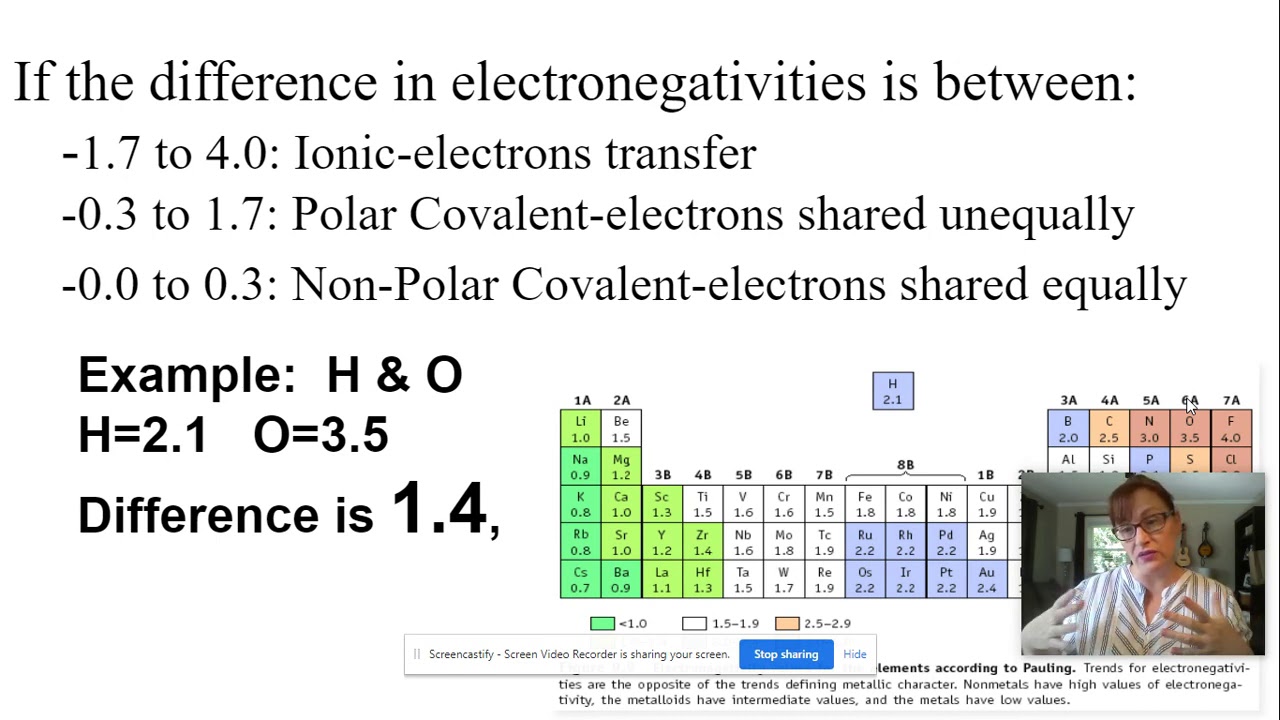
Electronegativity Difference Example
Images related to the topicElectronegativity Difference Example
What is the electronegativity of RB?
The first scale of electronegativity was developed by Linus Pauling and on his scale rubidium has a value of 0.82 on a scale running from from about 0.7 (an estimate for francium) to 2.20 (for hydrogen) to 3.98 (fluorine).
What is the electronegativity difference between sodium and fluorine?
The difference between the electronegativity value of fluorine and sodium is more than 1.7 so the compound formed by them will be ionic in nature. The difference between the electronegativity value of fluorine and sodium is more than 1.7 so the compound formed by them will be ionic in nature.
Related searches to What is the electronegativity difference of Na and Br?
- electronegativity difference between sodium and bromine
- sodium bromide electronegativity value
- electronegativity value of sodium
- what is the electronegativity difference between na and cl
- what is the difference between the electronegativity values of sodium and bromine
- what is the electronegativity difference of na and br brainly
- electronegativity difference chart
- sodium bromide ionic or covalent
- sodium bromide electronegativity
- k and f electronegativity difference
- electronegativity difference of bromine
- what is the electronegativity difference of na and br
- what is the electronegativity difference between na and f
Information related to the topic What is the electronegativity difference of Na and Br?
Here are the search results of the thread What is the electronegativity difference of Na and Br? from Bing. You can read more if you want.
You have just come across an article on the topic What is the electronegativity difference of Na and Br?. If you found this article useful, please share it. Thank you very much.