Are you looking for an answer to the topic “What is the electronic configuration of Lithium has 3 electrons?“? We answer all your questions at the website Ecurrencythailand.com in category: +15 Marketing Blog Post Ideas And Topics For You. You will find the answer right below.
Keep Reading

Table of Contents
What is the electronic configuration of lithium with 3 electrons?
Does lithium have 3 electrons?
Lithium is an alkali metal with the atomic number = 3 and an atomic mass of 6.941 g/mol. This means that lithium has 3 protons, 3 electrons and 4 neutrons (6.941 – 3 = ~4).
Lithium Electron Configuration
Images related to the topicLithium Electron Configuration

What is the charge of lithium with 3 electrons?
For the lithium atom, Z=3 ; there are 3 positive, nuclear charges. There are 3 extra-nuclear electronic charges, i.e. borne by the 3 electrons, in the neutral atom. Lithium generally loses one electron to form the Li+ cation.
What element has 3 electron orbitals?
For instance, lithium ( Listart text, L, i, end text) has three electrons: two fill the 1 s 1s 1s orbital, and the third is placed in the 2 s 2s 2s orbital, giving an electron configuration of 1 s 2 1s^ 2 1s21, s, squared 2 s 1 2s^ 1 2s12, s, start superscript, 1, end superscript.
What is the electron configuration 1s2 2s2 2p6?
The electron configuration for Copper (Co) is: 1s2 2s2 2p6 3s3 3p6 4s2 3d7. A chemist would shorten this notation to just “3d7” – calling Copper by the subshell of highest energy that contains any electrons.
What is 1s 2s 2p 3s 3p?
1s 2s 2p 3s 3p represents the electron orbital energy levels.
How many electron configurations are in lithium?
Lithium is the third element with a total of 3 electrons. In writing the electron configuration for lithium the first two electrons will go in the 1s orbital. Since 1s can only hold two electrons the remaining electron for Li goes in the 2s orbital. Therefore the Li electron configuration will be 1s22s1.
See some more details on the topic What is the electronic configuration of Lithium has 3 electrons? here:
Electron Configuration for Lithium (Li) – TerpConnect
Lithium is the third element with a total of 3 electrons. In writing the electron configuration for lithium the first two electrons will go in the 1s …
Lithium(Li) electron configuration and orbital diagram
Lithium is the 3rd element in the periodic table and its symbol is ‘Li’. The total number of electrons in lithium is three. These electrons are arranged …
What is the complete electron configuration of lithium? – Quora
Lithium is element 3, so it has 3 electrons, and they would be arranged as: 1s:2, 2s:1. That one 2s electron is the valence electron, giving Li a valence of …
Lithium Orbital diagram, Electron configuration, and Valence …
Lithium Electron configuration using the Aufbau Principle. A Lithium atom is a neutral atom that has 3 atomic numbers which imply it has a total of 3 electrons.
How many electrons are there in Li+?
However, in this question we are being asked the electrons in a lithium ion. Lithium, as a metal, donates electrons and so must have lost an ion in order to have a +1 charge. Therefore, the number of electrons must be 2.
What is Li charge?
lithium ion. lithium cation. Lithium(1+) Lithium, ion (Li1+)
Which element forms an ion with charge 3+?
Knowing this lets us use the periodic table to identify the element as Al (aluminum). The Al atom has lost three electrons and thus has three more positive charges (13) than it has electrons (10). This is the aluminum cation, Al3+.
How many electrons does cr3+ have?
It has a total of 24 electrons.
Li+ Electron Configuration (Lithium Ion)
Images related to the topicLi+ Electron Configuration (Lithium Ion)

What is the electron configuration chart?
The electron configuration of an atom of any element is the of electrons per sublevel of the energy levels of an atom in its ground state. This handy chart compiles the electron configurations of the elements up through number 104.
What element that has electron configurations of 1s2 2s2 2p6 3s1?
| A | B |
|---|---|
| Oxygen | 1s2 2s2 2p4 |
| Fluorine | 1s2 2s2 2p5 |
| Neon | 1s2 2s2 2p6 |
| Sodium | 1s2 2s2 2p6 3s1 |
What element is 1s2 2s2 2p6 3s2 3p6 4s2 3d10?
| A | B |
|---|---|
| calcium | 1s2 2s2 2p6 3s2 3p6 4s2 |
| chromium | 1s2 2s2 2p6 3s2 3p6 4s1 3d5 ! |
| copper | 1s2 2s2 2p6 3s2 3p6 4s1 3d 10 ! |
| bromine | 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p5 |
What is 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6?
Kr (Krypton) 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6. Rb (Rubidium) 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s1. Sr (Strontium)
What element is 1s2 2s2 2p6 3s2 3p6 4s2 3d2?
If you are referring to a neutral atom, then Vanadium (V) has that particular electron configuration.
How many electrons does 3p have?
1 Answer. 3p contains 6 electrons.
How many electrons can 3s hold?
| Shell name | Subshell name | Subshell max electrons |
|---|---|---|
| M | 3s | 2 |
| 3p | 6 | |
| 3d | 10 | |
| N | 4s | 2 |
What is meant by 1s 2s 3s?
In the question 1s 2s 2p 3s 3p represents electron orbital energy levels. These orbital energy levels depend on 2 quantum numbers-Principal quantum number (n) and Azimuthal quantum number(l) .
What is the molecular configuration of lithium?
…
The Diatomic Molecules of the Second Period.
| Molecule | Electron Configuration | Bond Order |
|---|---|---|
| Li2 | (σ2s)2 | 1 |
| Be2 (unstable) | (σ2s)2(σ∗2s)2 | 0 |
| B2 | (σ2s)2(σ∗2s)2(π2py,π2pz)2 | 1 |
| C2 | (σ2s)2(σ∗2s)2(π2py,π2pz)4 | 2 |
Electron Configuration – Basic introduction
Images related to the topicElectron Configuration – Basic introduction
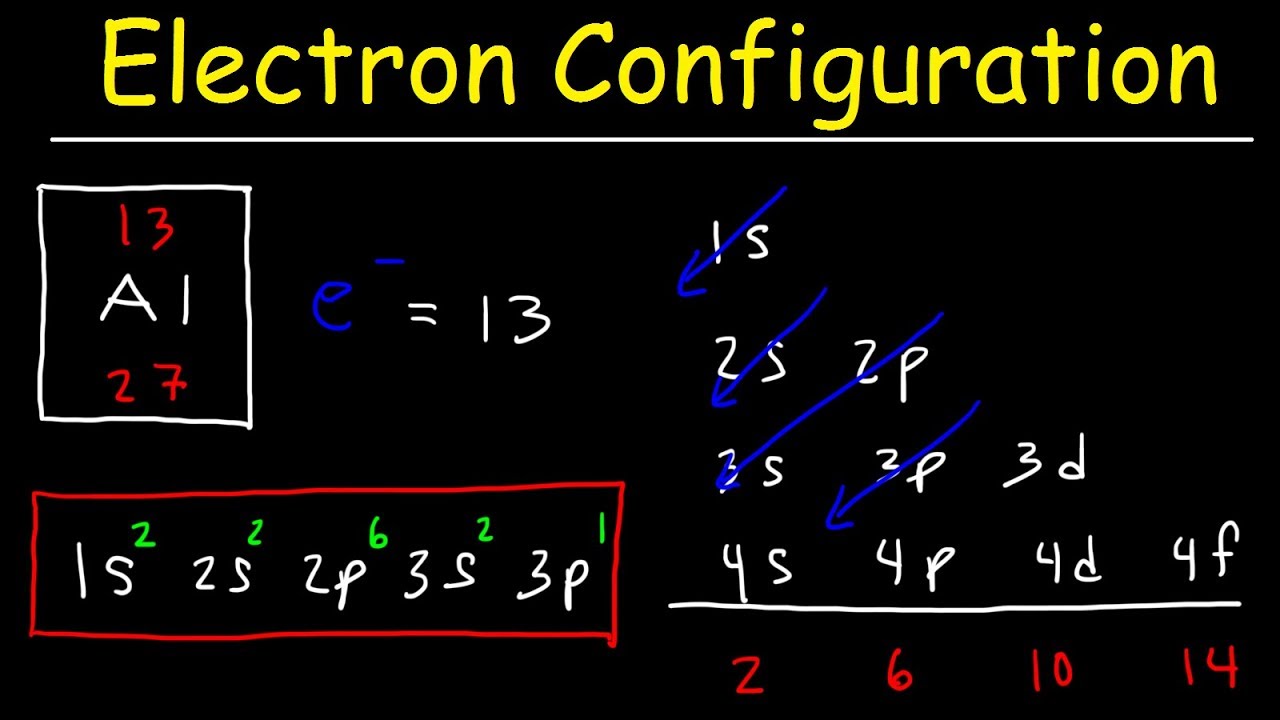
How do you find the electron configuration?
To calculate an electron configuration, divide the periodic table into sections to represent the atomic orbitals, the regions where electrons are contained. Groups one and two are the s-block, three through 12 represent the d-block, 13 to 18 are the p-block and the two rows at the bottom are the f-block.
How many valence electrons does Li+ have?
The electron configuration shows that the lithium atom has acquired the electron configuration of helium. Since the last shell of a lithium-ion has two electrons, the valence electrons of a lithium-ion are two.
Related searches to What is the electronic configuration of Lithium has 3 electrons?
- what is the electronic configuration of lithium 3 electrons
- what is the electronic configuration of lithium has 3 electrons
- Write the electron configuration for f
- boron has 5 electrons which of the following below is bronze electronic configuration
- boron has 5 electrons which of the following below is borons electronic configuration
- lithium has three electrons
- boron has 5 electrons which of the following below is boron’s electronic configuration
- write the electron configuration for f
- boron has 5 electrons which of the following below is barons electronic configuration
- what is the electronic configuration of lithium brainly
- what is the electronic configuration of boron
Information related to the topic What is the electronic configuration of Lithium has 3 electrons?
Here are the search results of the thread What is the electronic configuration of Lithium has 3 electrons? from Bing. You can read more if you want.
You have just come across an article on the topic What is the electronic configuration of Lithium has 3 electrons?. If you found this article useful, please share it. Thank you very much.
