Are you looking for an answer to the topic “What is the electrostatic potential energy between an electron and a proton?“? We answer all your questions at the website Ecurrencythailand.com in category: +15 Marketing Blog Post Ideas And Topics For You. You will find the answer right below.
The potential energy of the electron in the field of the positive proton point charge is U(r) = -qeV(r) = – keqe2/r. The total energy is the sum of the electron’s kinetic energy and its potential energy.The electrostatic potential energy between proton and electron separated by a distance 1 A0 is: (A) 13.6 eV.A proton and an electron have same kinetic energy. Since the mass of proton is higher than electron, we can say proton has more energy than electron.

What is the electric potential energy between proton and electron?
The electrostatic potential energy between proton and electron separated by a distance 1 A0 is: (A) 13.6 eV.
Why is potential energy of proton greater than electron?
A proton and an electron have same kinetic energy. Since the mass of proton is higher than electron, we can say proton has more energy than electron.
The electrostatic potential energy between proton and electron separated by a distance 1 Å is
Images related to the topicThe electrostatic potential energy between proton and electron separated by a distance 1 Å is

What is the electrostatic potential energy of an electron in hydrogen atom?
In hydrogen atom electron of charge −e and mass m revolves round the nucleus in a circular orbit of radius r. The electrostatic potential energy of the electron is 4πε01 times.
What is potential for a system of electron and proton placed as separation of 1 Metre?
6×10−19J)
How do you find the electric potential energy of an electron?
The potential energy of the electron in the field of the positive proton point charge is U(r) = -qeV(r) = – keqe2/r. The total energy is the sum of the electron’s kinetic energy and its potential energy.
Is electric potential energy positive or negative?
Note that the electrical potential energy is positive if the two charges are of the same type, either positive or negative, and negative if the two charges are of opposite types.
How does the potential energy change when an electron and a proton become further apart?
However, the potential energy will decrease when a proton and an electron are brought nearer. Work will be done by the force of attraction between them.
See some more details on the topic What is the electrostatic potential energy between an electron and a proton? here:
The elcectro static potential energy between proton and …
The electro static potential energy between proton and electron distance =1A0=1×10−10m. U=4πϵ01rq1q2. =10−109×109×1.6×10−19×1.6×10−19. =13.6eV ∵ (1e …
The electrostatic potential energy between proton and class …
The electrostatic potential energy between proton and electron separated by a distance 1 A0 is A 136 eV B 136 eV C 144 eV D 144 eV.
Electric potential (article) | Khan Academy
But the basic rules for electric forces are surprisingly simple: electrons repel other electrons, but protons and electrons attract each other. Here we’ll talk …
How did the electric potential energy of the proton change during this motion give your explanation on the energy change?
Solution : The potential enerfy of the proton decreases (as it gains kinetic energy due to its motion in the electric field) Further the proton (being positive) moves from higher to lower electric potential.
What happens when an electron and proton get closer together?
That is, a proton and an electron will attract each other. The closer they are together, the stronger this attraction will be. Two protons (or two electrons) will repel each other. And again, the closer together they are, the stronger the repulsion.
Why is the potential energy of a hydrogen atom negative?
The energy is negative due to the attractive nature of the Coulombic interaction. This is alternatively visualized as an atom whose electron has been moved infinitely far away. The potential energy of the electron is defined as zero as there is no interaction at infinite distance.
What is the energy of electron in hydrogen atom for n CC?
The energy of the electron of hydrogen atom in its nth orbit is given by En=−n213. 6 electron volt(eV).
What is the energy of an electron in a hydrogen atom at the second principal energy level?
Electrons in a hydrogen atom must be in one of the allowed energy levels. If an electron is in the first energy level, it must have exactly -13.6 eV of energy. If it is in the second energy level, it must have -3.4 eV of energy.
Electric Potential Energy
Images related to the topicElectric Potential Energy
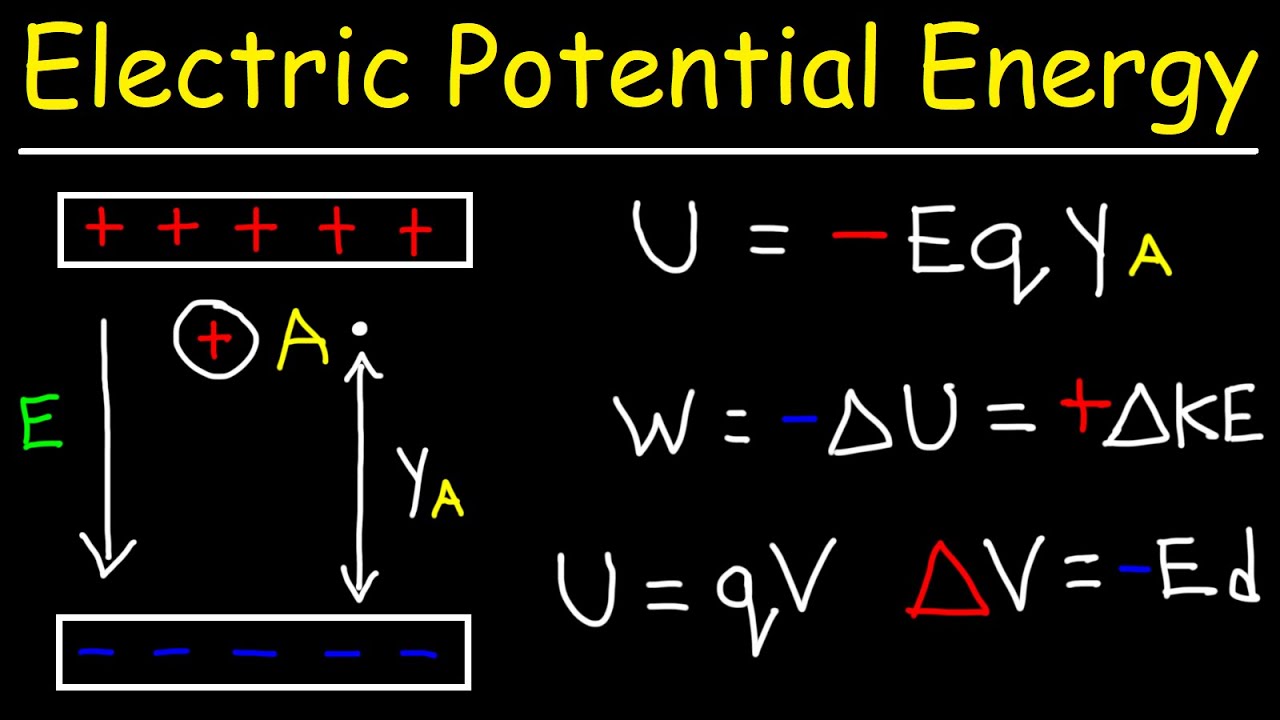
When a proton approaches another proton the electric potential energy?
Electrostatic potential energy is the capacity to do work which arises from the position or configuration. As only little work is needed for approaching an electron to proton, their electrostatic potential energy decreases.
How do you calculate the electric potential energy between two charges?
V = k × [q/r]
Where, V = electric potential energy. q = point charge. r = distance between any point around the charge to the point charge.
How do you find the electric potential difference between two points?
In a uniform electric field, the equation to calculate the electric potential difference is super easy: V = Ed. In this equation, V is the potential difference in volts, E is the electric field strength (in newtons per coulomb), and d is the distance between the two points (in meters).
What is the potential energy of a proton?
The potential energy of a proton is 3. 2×10−18J at a particular point. The electric potential at this point is: (Given charge on a proton is 1.
What is the potential from a proton?
And, this is going to be 8.99 times ten to the nine Newton meters squared per Coulombs squared times 1.60 times ten to the minus 19 Coulombs, because that’s the charge of the proton. And, divide by 0.530 times ten to the minus ten meters, giving us a potential of 27.1 volts.
Why is potential energy of electron negative?
when that electron comes close to the nucleus ,the stability increases due to the loss of energy of electron and thus the energy of electron becomes less negative. That’s why the electrons energy is taken as negative. Was this answer helpful?
What is the difference between electrostatic potential and electrostatic potential energy?
The basic difference between electric potential and electric potential energy is that Electric potential at a point in an electric field is the amount of work done to bring the unit positive charge from infinity to that point, while electric potential energy is the energy that is needed to move a charge against the …
Why is electrostatic potential energy of a pair of like point charges positive?
Now since, we are talking about like charges, the force will be repulsive when the charges are brought closer. So we will have to do the work to bring them closer. Hence the electrostatic potential energy will also be positive.
Is electrostatic energy always positive?
For like charges, the potential energy is always positive, that is because we need to put energy in the system to bring like charges closer together.
When an electron and proton are brought closer the electrostatic potential energy of the system?
There is attractive force between an electron and a proton, therefore when they come nearer, the work is done by the system itself and so the potential energy of system decreases.
MCQ14 POTENTIAL CAPACITANCE Class 12 Term 1 The electrostatic potential energy between pr
Images related to the topicMCQ14 POTENTIAL CAPACITANCE Class 12 Term 1 The electrostatic potential energy between pr

When one electron is taken towards the other electron then the electric potential energy of the system?
when an electron is brought near another electron, the work has to be done against the repulsive force since like charges repel each other. This work done is stored in the form of electrostatic potential energy and hence the electric potential energy of the system increases. Hope it helps!
When two protons are brought close to each other then potential energy?
If two protons are brought near one another, the potential energy of the system will increase.
Related searches to What is the electrostatic potential energy between an electron and a proton?
- what is the electrostatic potential energy in joules between an electron and a proton
- potential energy of proton formula
- what is the electrostatic potential energy between two protons that are separated by 62 pm
- what is the electrostatic potential energy (in joules) between an electron and a proton
Information related to the topic What is the electrostatic potential energy between an electron and a proton?
Here are the search results of the thread What is the electrostatic potential energy between an electron and a proton? from Bing. You can read more if you want.
You have just come across an article on the topic What is the electrostatic potential energy between an electron and a proton?. If you found this article useful, please share it. Thank you very much.