Are you looking for an answer to the topic “What is the equilibrium constant for the reaction H2 g )+ I2 G ⇌ 2HI G at 700 K?“? We answer all your questions at the website Ecurrencythailand.com in category: +15 Marketing Blog Post Ideas And Topics For You. You will find the answer right below.
H2 (g) + I2 (g) ↔ 2HI (g) is 54.8.The equilibrium constant for the reaction, H2(g) +12(g) 2HI(g) is 32 at a given temperature. The equilibrium concentration of 1, and HI are 0.5x 10-and 8×10- M respectively.The value of the equilibrium constant, Kc, for the reaction H2(g) + I2(g) ⇔ 2HI (g) at 448°C is 50.5. What are the concentrations of H2, I2 and HI at equilibrium? Solution: In this case we are not given any of the equilibrium concentrations.

Table of Contents
What is the equilibrium constant for the reaction H2 g )+ I2 G ⇌ 2HI G?
The equilibrium constant for the reaction, H2(g) +12(g) 2HI(g) is 32 at a given temperature. The equilibrium concentration of 1, and HI are 0.5x 10-and 8×10- M respectively.
What is the KC of H2 I2 ⇌ 2HI?
The value of the equilibrium constant, Kc, for the reaction H2(g) + I2(g) ⇔ 2HI (g) at 448°C is 50.5. What are the concentrations of H2, I2 and HI at equilibrium? Solution: In this case we are not given any of the equilibrium concentrations.
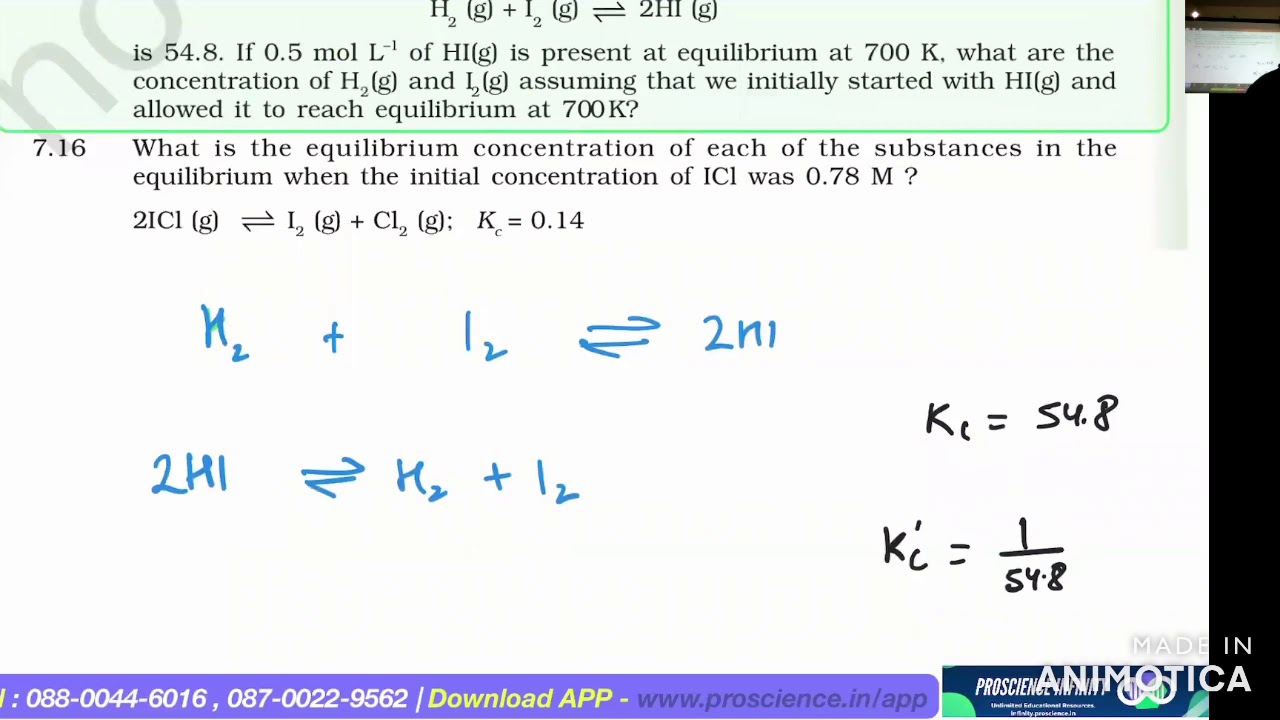
At 700K, equilibrium constant for the reaction: H2 (g) + I2 (g) ⇌ 2HI (g)is 54.8…
Images related to the topicAt 700K, equilibrium constant for the reaction: H2 (g) + I2 (g) ⇌ 2HI (g)is 54.8…

What is the value of the equilibrium constant KC at 700 K?
At 700 K, the equilibrium constant for the reaction; H2(g)+I2(g)⇌2HI(g) is 54.
What is the order of reaction H2 I2 2HI?
It is a 2nd order reaction.
What is the equilibrium expression for the reaction?
Since kf and kr are constants, the ratio of kf divided by kr must also be a constant. This ratio is the equilibrium constant for the reaction, Kc. The ratio of the concentrations of the reactants and products is known as the equilibrium constant expression.
What kind of equilibrium does the reaction below show H2 G +I2 G ↔ 2HI G?
H2(g) +I2(g) ↔ 2HI(g) Chemical Equilibrium. Heterogeneous equilibrium, all the reactants and products are in the same physical state.
How do you calculate equilibrium KC?
Multiply concentrations of CO2 and H2O to get Kc. An important rule is that all components which are in the solid state are not included in the equilibrium constant equation. Thus, in this case, Kc=[CO2] x [H2O]=1.8 mole/L x 1.5 mole/L=2.7 mole^2/L^2.
See some more details on the topic What is the equilibrium constant for the reaction H2 g )+ I2 G ⇌ 2HI G at 700 K? here:
At 700 K the equilibrium constant for the reaction: H2(g) + I2(g …
Let the concentration of H2(g) and I2(g) at equilibrium be x mol L-1. Then for the reaction. H2(g) + I2(g) ⇌ 2HI(g).
At 700K, equilibrium constant for the reaction – Instasolv
Check here step-by-step solution of ‘At 700K, equilibrium constant for the reaction: H2(g)+I2(g)=2HI(g) is 54.8 . If 0.5molL^−1 of HI (g) is’ questions at …
Key Worksheet Chemical Equilibrium
Calculate the equilibrium constant for the reaction: A g) + 2B (g). 30 g … a) At a certain temperature, Kc is 4.13 x 10 -2 for the equilibrium: 2 1Br (9).
What is the relationship between KP and KC for the reaction H2 I2 2HI?
For the reaction H2 + I2 2HI, Kp = Kc .
What will the rate constant be for this reaction at 700 K?
The rate constant of a reaction at 700 K and 760 K are 0.011 M^-1 s^-1 , respectively.
At `700 K` equilibrium constant for the reaction, `H_(2(g))+I_(2(g))hArr2HI_((g))`
Images related to the topicAt `700 K` equilibrium constant for the reaction, `H_(2(g))+I_(2(g))hArr2HI_((g))`

What type of reaction is H2 I2 → 2HI?
Answer: It is a combination reaction.
Under what condition the order of reaction 2HI gives H2 I2 is zero?
is of zero-order in which HI is present at high partial pressure.
What is the reaction order of H2?
2 H2O + N2
The reaction is first order in [ H2 ] and second order in [ NO ]. The overall order of this reaction is three; (the sum of all the individual orders). Note that the products do not (usually) appear in the rate law.
What is equilibrium constant KC?
For example, the equilibrium constant of concentration (denoted by Kc) of a chemical reaction at equilibrium can be defined as the ratio of the concentration of products to the concentration of the reactants, each raised to their respective stoichiometric coefficients.
What is the equilibrium constant for the given reaction?
The equilibrium constant is equal to the rate constant for the forward reaction divided by the rate constant for the reverse reaction.
What is the equilibrium expression for the given reaction SrCO3 G <-- --> SRO’s CO2 g?
For the reaction, SrCO3(s) =sro(s) +co2(g), the value of equilibrium constant Kp =2.2×10-4at 1002 k.
What is the effect of pressure in the equilibrium system H2 G I2 G ⬄ 2HI G?
There is no change in the number of moles of gaseous species. Hence, the change in pressure has no effect on the equilibrium.
7.15 At 700 K, equilibrium constant for the reaction: H2 (g) + I 2(g) ⇌ 2HI – class11equilibrium
Images related to the topic7.15 At 700 K, equilibrium constant for the reaction: H2 (g) + I 2(g) ⇌ 2HI – class11equilibrium
What are the equilibrium concentrations of H2 l2 and hi if the initial concentration of hi was 4.0 m and the initial concentrations of both H2 and l2 are zero?
Under certain conditions, the equilibrium constant for the following equation is 6.0. What are the equilibrium concentrations of H2,l2, and HI if the initial concentration of HI was 4.0 M and the initial concentrations of both H2 and l2 are zero? The equilibrium constant for the reaction below is 4.0.
What is the concentration of I2 at equilibrium?
The equilibrium concentration of I2 is 0.0300 M .
Related searches to What is the equilibrium constant for the reaction H2 g )+ I2 G ⇌ 2HI G at 700 K?
- given that at 700 k kp 54 0 for the reaction
- what is the value of kp for the reaction so3(g)←→so2(g)+12o2(g)
- calculate the value of kc at 700 k for the equilibrium
- the following reaction has kc 57 at 700 k h2 g i2 g ⇄ 2hi g what is kp at the same temperature
- h2 + i2 2hi equilibrium constant
- the following reaction has kc 57 at 700 k h2 g i2 g 2hi g what is kp at the same temperature
- what is the equilibrium constant for the reaction h2(g)+i2(g)⇌2hi(g)
- what is the value of kp for the reaction so3gso2g12o2g
- h2 i2 2hi equilibrium constant
- h2g i2g2hig equilibrium constant
- h2(g) + i2(g)⇌2hi(g equilibrium constant)
Information related to the topic What is the equilibrium constant for the reaction H2 g )+ I2 G ⇌ 2HI G at 700 K?
Here are the search results of the thread What is the equilibrium constant for the reaction H2 g )+ I2 G ⇌ 2HI G at 700 K? from Bing. You can read more if you want.
You have just come across an article on the topic What is the equilibrium constant for the reaction H2 g )+ I2 G ⇌ 2HI G at 700 K?. If you found this article useful, please share it. Thank you very much.
