Are you looking for an answer to the topic “What is the multiplicity of a molecule?“? We answer all your questions at the website Ecurrencythailand.com in category: +15 Marketing Blog Post Ideas And Topics For You. You will find the answer right below.
The multiplicity of a molecule is determined by the number of unpaired electrons that it contains. Most normal ground-state equilibrium structures consist solely of electron pairs and are called “singlets”. We say that these systems are “closed-shell molecules”.Spin multiplicity relation (2S+1) will be useful to find the multiplicity of a molecule. You have to arrange the electron properly and find how many unpaired electrons are available (each one has +1/2). If no unpaired electron in a molecule then multiplicity will become 1(singlet).Multiplicity (chemistry) Multiplicity in quantum chemistry is used to distinguish between several degenerate wavefunctions that differ only in the orientation of their angular spin momenta. It is defined as 2S+1, where S is the angular spin momentum.
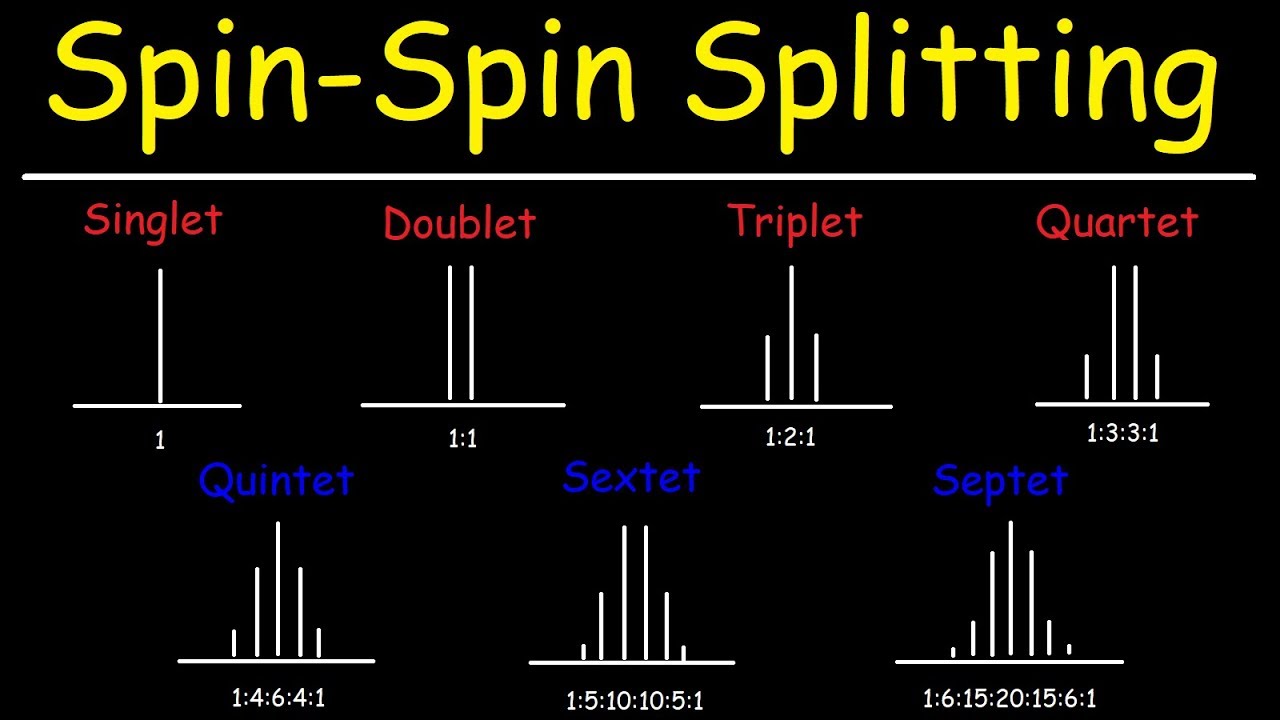
How do you find the multiplicity of a molecule?
Spin multiplicity relation (2S+1) will be useful to find the multiplicity of a molecule. You have to arrange the electron properly and find how many unpaired electrons are available (each one has +1/2). If no unpaired electron in a molecule then multiplicity will become 1(singlet).
What is the multiplicity of a reaction?
Multiplicity (chemistry) Multiplicity in quantum chemistry is used to distinguish between several degenerate wavefunctions that differ only in the orientation of their angular spin momenta. It is defined as 2S+1, where S is the angular spin momentum.
Spin Spin Splitting – N+1 Rule – Multiplicity – Proton NMR Spectroscopy
Images related to the topicSpin Spin Splitting – N+1 Rule – Multiplicity – Proton NMR Spectroscopy

What is meant by multiplicity of an atom?
the number of possible spatial orientations of the total spin of an atom or molecule.
How do you find the charge and multiplicity?
Charge is the overall charge of the molecular system and multiplicity describes how the electrons of the system exists. Multiplicity is mathematically defined as 2S+1 = spin multiplicity, where S equals the total angular momentum of the unbound electrons.
How do you find the multiplicity of an electron configuration?
…
Species having unpaired electrons in both mixed alignment (↑)(↓)
| Spin multiplicity value | Spin state |
|---|---|
| 1 | Singlet |
| 2 | Doublet |
| 3 | Triplet |
| 4 | Quartet |
What is meant by multiplicity of spectral lines?
Answer: the number of possible spatial orientations of the total spin of an atom or molecule.
What is the spin multiplicity of nitrogen atom?
spin multiplicity=2s+1=2(3×21)+1=4.
See some more details on the topic What is the multiplicity of a molecule? here:
Multiplicity (chemistry) – Wikipedia
In spectroscopy and quantum chemistry, the multiplicity of an energy level is defined as 2S+1, where S is the total spin angular momentum.
Multiplicity (chemistry) – Academic Dictionaries and …
When all electrons are paired S = 0, and the multiplicity = 2(0) + 1 = 1. This case is called a singlet. If a molecule has 1 unpaired electron S = +½ and 2S + 1 …
What is the multiplicity of an atom? – Easierwithpractice.com
The molecule now has one electron in the lower orbital and one in an upper orbital, these form the excited state, and if the spins are paired it …
Difference Between Multiplicity and Bond Order
Multiplicity refers to the number of possible orientations of the spin of energy level. This concept is useful in spectroscopy and quantum …
What is singlet and triplet state?
Singlet state: All electrons in the molecule are spin paired. It is called a singlet because there is only one possible orientation in space. Triplet state: One set of electron spins is unpaired. It is called a triplet because there are three possible orientations in space with respect to the axis.
How do you find the spin multiplicity of oxygen?
1 The multiplicity is given by 2S+1, where S is the spin. The spin of an electron is (+/-) 1/2. oxygen is a bi-radical.
Multiplicity: n + 1 rule | Spectroscopy | Organic chemistry | Khan Academy
Images related to the topicMultiplicity: n + 1 rule | Spectroscopy | Organic chemistry | Khan Academy

What is bond multiplicity?
The multiplicity of a chemical bond is determined by the number of electron pairs that occupy the region between the two bonded atoms in bonding molecular orbitals. The hydrogen molecule has, for example, a single bond with two electrons in one orbital formed from the 1s orbitals on each atom.
What is the spin multiplicity of a radical?
Spin multiplicity is based on the number of unpaired electron, =2S+1. Where S=n(1/2).
What is the multiplicity of ammonia?
NH3 is a neutral molecule and has 10 (7+1+1+1) electrons. Therefore the charge/multiplicity entry is 0 1.
How do you find the multiplicity of a Gaussian?
Spin multiplicity is based on the number of unpaired electron, =2S+1. Where S=n(1/2).
What is the spin multiplicity of Oxygen dimer o2 2?
With 2 electrons to be placed in 2 degenerate orbitals, a number of variations are possible and the arrangement above where the 2 electrons are parallel is considered to be the most stable. Note that the spin multiplicity is given by the formula, 2S+1 and so for S=1 from s=½ + ½ then 2S+1 = 3 i.e. a spin triplet.
What is multiplicity order?
In complex analysis
Then the power series of f about z0 begins with the nth term, and f is said to have a root of multiplicity (or “order”) n. If n = 1, the root is called a simple root. We can also define the multiplicity of the zeroes and poles of a meromorphic function.
What is a root multiplicity?
A multiple root is a root with multiplicity , also called a multiple point or repeated root. For example, in the equation. , 1 is multiple (double) root. If a polynomial has a multiple root, its derivative also shares that root.
What is the multiplicity of nitrogen?
Multiplicity of nitrogen in ground state
It indicates the number of protons and number of electrons both is equal to 7. Nitrogen has 3 unpaired electrons in its outermost shell.
Spin Multiplicity | Concept, Trick Questions in 1 Video | JEE NEET | by Sarvesh Kumar
Images related to the topicSpin Multiplicity | Concept, Trick Questions in 1 Video | JEE NEET | by Sarvesh Kumar

What is the formula of spin multiplicity?
> The formula used for calculating spin multiplicity is 2S+1, Where, S= 2xmaximum number of unpaired electrons in 4d orbital x12. .
How do you find the spin multiplicity of a coordination compound?
All Answers (4) Spin multiplicity is given by 2S + 1, where S is the total electron spin for the molecule. Paired electrons have a net zero spin and do not contribute. For one unpaired electron (of spin 1/2), 2(1/2) + 1 = 2; the species is a doublet.
Related searches to What is the multiplicity of a molecule?
- what is the multiplicity of a molecule of carbon
- spin multiplicity of o2
- spin multiplicity of n2
- hydroxide ion multiplicity
- what is the multiplicity of a molecule calculator
- what is the multiplicity of a molecule of water
- how to calculate multiplicity in nmr
- what is multiplicity in chemistry
- spin multiplicity rule
- spin multiplicity of a radical
- multiplicity physics
Information related to the topic What is the multiplicity of a molecule?
Here are the search results of the thread What is the multiplicity of a molecule? from Bing. You can read more if you want.
You have just come across an article on the topic What is the multiplicity of a molecule?. If you found this article useful, please share it. Thank you very much.