Are you looking for an answer to the topic “What is the natural abundance of silicon 30?“? We answer all your questions at the website Ecurrencythailand.com in category: +15 Marketing Blog Post Ideas And Topics For You. You will find the answer right below.
Natural abundance of Si-28 is 92.23% and of Si-30 is 3.1%.The relative abundance of 28Si, 29Si and 30Si is 92.23%, 4.67% and 3.10%, respectively. By the1920s, all three stable silicon isotopes had been discovered. Mass spectrometric studies on silicon isotope variation in the natural environment started in the 1950s.The abundance of Si-28 is 92.23%. Si-29 is 4.68% and Si-30 is 3.09%. Because most Si atoms have a mass of 28 amu, the average mass of all silicon atoms is very close to 28.

What is the percent abundance of silicon?
The relative abundance of 28Si, 29Si and 30Si is 92.23%, 4.67% and 3.10%, respectively. By the1920s, all three stable silicon isotopes had been discovered. Mass spectrometric studies on silicon isotope variation in the natural environment started in the 1950s.
What is the abundance of silicon 28 29 30?
The abundance of Si-28 is 92.23%. Si-29 is 4.68% and Si-30 is 3.09%. Because most Si atoms have a mass of 28 amu, the average mass of all silicon atoms is very close to 28.
Physical Structure of Atoms: Calculate the Atomic Mass of Naturally Occurring Silicon
Images related to the topicPhysical Structure of Atoms: Calculate the Atomic Mass of Naturally Occurring Silicon

What does Silicon-30 contain?
Please visit the Silicon element page for information specific to the chemical element of the periodic table. Silicon-30 atom is the stable isotope of silicon with relative atomic mass 29.9737702. The least abundant (3.09 atom percent) isotope of naturally occurring silicon.
How do you calculate natural abundance?
The equation can be set up as a percent or as a decimal. As a percent, the equation would be: (x) + (100-x) = 100, where the 100 designates the total percent in nature. If you set the equation as a decimal, this means the abundance would be equal to 1. The equation would then become: x + (1 – x) = 1.
What is the percent abundance of silicon 29?
| Isotope | Decay | |
|---|---|---|
| abundance | mode | |
| 28Si | 92.2% | stable |
| 29Si | 4.7% | stable |
| 30Si | 3.1% | stable |
How many neutrons are in an atom of silicon-30?
How many neutrons does Silicon-30 have? 16 neutrons. Silicon-30 is an isotope of Silicon. It has a mass number of 30.
What is the percent abundance of an isotope?
Isotopes are atoms that have the same number of protons but different numbers of neutrons. The atomic masses of isotopes differ. The percentage of atoms with a specific atomic mass found in a naturally occurring sample of an element is known as its relative abundance.
See some more details on the topic What is the natural abundance of silicon 30? here:
Silicon-30 atom | Si – PubChem
Silicon-30 atom is the stable isotope of silicon with relative atomic mass 29.9737702. The least abundant (3.09 atom percent) isotope of naturally occurring …
Isotopes of silicon – Wikipedia
Si (the most abundant isotope, at 92.23%), 29Si (4.67%), and 30Si (3.1%) are stable. The longest-lived radioisotope is 32Si, which is produced by cosmic ray …
silicon-30 atom (CHEBI:37976)
The stable isotope of silicon with relative atomic mass 29.9737702. The least abundant (3.09 atom percent) isotope of naturally occurring silicon. Stars, This …
Silicon-30 Isotope | AMERICAN ELEMENTS ®
Silicon-30) is a stable (non-radioactive) isotope of Silicon. It is both naturally occurring and produced by fission. Silicon-30 is one of over 250 stable …
Which isotope of silicon is most abundant?
Silicon has nine isotopes, with mass numbers from 25-33. Si (the most abundant isotope, at 92.23%), 29Si (4.67%), and 30Si (3.1%) are stable; 32Si is a radioactive isotope produced by argon decay.
What is the mass number of silicon?
How To Find The Percent Abundance of Each Isotope – Chemistry
Images related to the topicHow To Find The Percent Abundance of Each Isotope – Chemistry
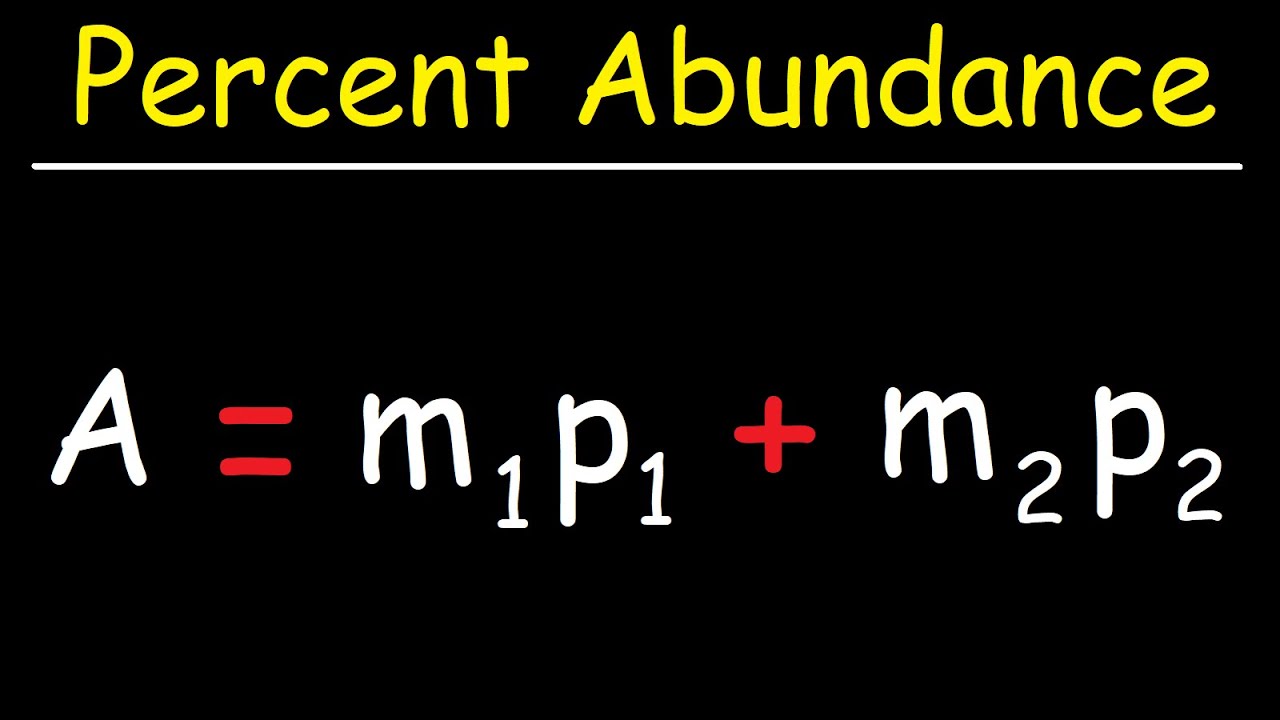
What is the average atomic mass of silicon 28 29 30?
There are three isotopes of silicon. They have mass numbers of 28, 29 and 30. The average atomic mass of silicon is 28.086amu.
What is the natural abundance of lithium 8?
Naturally occurring lithium is composed of two stable isotopes 6Li and 7Li, the latter being the more abundant (92.5% natural abundance). Seven radioisotopes have been characterized, the most stable being 8Li with a half-life of 838 ms and 9Li with a half-life of 178.3 ms.
How do you find the percent abundance of an isotope mass?
Step 1: List the known and unknown quantities and plan the problem. Change each percent abundance into decimal form by dividing by 100. Multiply this value by the atomic mass of that isotope. Add together for each isotope to get the average atomic mass.
How do you find the natural abundance of lithium?
…
| Atomic Mass = | [(mass of isotope) (%abundance) ] + [(mass of isotope) (%abundance)] + [….] |
|---|---|
| 100% |
What is the mass of silicon 28?
silicon-28 atom (CHEBI:37975) The stable isotope of silicon with relative atomic mass 27.9769265.
How many protons are in a silicon 29 isotope?
| Properties of Silicon-29 Isotope: | SILICON-29 |
|---|---|
| Nucleon Number (A) | 29 |
| Proton Number (Z) | 14 |
| Half-life | Stable |
| Spin | 0.5 |
How many neutrons are in silicone?
The average silicon atom has fourteen protons, fourteen electrons, and most have 14 neutrons.
How many protons are in 28si?
| Properties of Silicon-28 Isotope: | SILICON-28 |
|---|---|
| Nucleon Number (A) | 28 |
| Proton Number (Z) | 14 |
| Half-life | Stable |
| Spin | 0 |
How to Find the Abundance of Each Isotope
Images related to the topicHow to Find the Abundance of Each Isotope

How do you find natural abundance of isotopes?
To calculate the percent abundance of each isotope in a sample of an element, chemists usually divide the number of atoms of a particular isotope by the total number of atoms of all isotopes of that element and then multiply the result by 100.
What is percent abundance definition?
Percent abundance is the percentage amount of all naturally occurring isotopes of an element. Isotopes are atoms of the same element that have identical atomic numbers but different mass numbers. This means isotopes are atoms having the same number of protons in the atomic nucleus, but different numbers of neutrons.
Related searches to What is the natural abundance of silicon 30?
- silicon-30 protons neutrons electrons
- what is the natural abundance of silicon 30 protons neutrons electrons
- relative atomic mass of silicon 28 29 30 brainly
- what is the natural abundance of silicon 30 years
- what is the natural abundance of silicon 30 000
- what is the natural abundance of silicon 30 years ago
- silicon isotopes natural abundance
- silicon 30 protons neutrons electrons
- silicon 30 charge
- silicon has three naturally occurring isotopes which isotope is most abundant
- what is the relative atomic mass of silicon 28 29 30
- percentage abundance of silicon 28 29 30
- silicon 30 electrons
Information related to the topic What is the natural abundance of silicon 30?
Here are the search results of the thread What is the natural abundance of silicon 30? from Bing. You can read more if you want.
You have just come across an article on the topic What is the natural abundance of silicon 30?. If you found this article useful, please share it. Thank you very much.