Are you looking for an answer to the topic “What is the natural abundance of silicon?“? We answer all your questions at the website Ecurrencythailand.com in category: +15 Marketing Blog Post Ideas And Topics For You. You will find the answer right below.
Silicon makes up 27.7% of the Earth’s crust by mass and is the second most abundant element (oxygen is the first). It does not occur uncombined in nature but occurs chiefly as the oxide (silica) and as silicates.Silicon, atomic number 14, has 24 known isotopes with mass numbers ranging from 22 to 45. Silicon has three stable iso- topes: 28Si with 92.23 % abundance, 29Si with 4.67 % abun- dance, and 30Si with 3.1 % abundance.The abundance of Si-28 is 92.23%. Si-29 is 4.68% and Si-30 is 3.09%. Because most Si atoms have a mass of 28 amu, the average mass of all silicon atoms is very close to 28.

What is the abundance of silicon isotopes?
Silicon, atomic number 14, has 24 known isotopes with mass numbers ranging from 22 to 45. Silicon has three stable iso- topes: 28Si with 92.23 % abundance, 29Si with 4.67 % abun- dance, and 30Si with 3.1 % abundance.
What is the abundance of silicon-28 29 30?
The abundance of Si-28 is 92.23%. Si-29 is 4.68% and Si-30 is 3.09%. Because most Si atoms have a mass of 28 amu, the average mass of all silicon atoms is very close to 28.
Physical Structure of Atoms: Calculate the Atomic Mass of Naturally Occurring Silicon
Images related to the topicPhysical Structure of Atoms: Calculate the Atomic Mass of Naturally Occurring Silicon

What is the natural abundance of Si-28?
Natural abundance of Si-28 is 92.23% and of Si-30 is 3.1%.
Which isotope of silicon is most abundant in nature?
Silicon (14Si) has 23 known isotopes, with mass numbers ranging from 22 to 44. Si (the most abundant isotope, at 92.23%), 29Si (4.67%), and 30Si (3.1%) are stable.
How do you find the abundance of isotopes?
To calculate the percent abundance of each isotope in a sample of an element, chemists usually divide the number of atoms of a particular isotope by the total number of atoms of all isotopes of that element and then multiply the result by 100.
How many isotopes of silicon exist?
Periodic Table–Silicon. Silicon has nine isotopes, with mass numbers from 25-33. Si (the most abundant isotope, at 92.23%), 29Si (4.67%), and 30Si (3.1%) are stable; 32Si is a radioactive isotope produced by argon decay.
How do you calculate percent abundance of silicon?
…
Calculate the isotopic abundances when given the average atomic weight and the isotopic weights.
| Isotope | Atomic Weight | Percent Abundance |
|---|---|---|
| Fe-54 | 53.9396 | 5.845 |
See some more details on the topic What is the natural abundance of silicon? here:
Isotopes of silicon – Wikipedia
Silicon (14Si) has 23 known isotopes, with mass numbers ranging from 22 to 44. … Si (the most abundant isotope, at 92.23%), 29Si (4.67%), and 30Si (3.1%) are …
Silicon Isotopes – an overview | ScienceDirect Topics
The relative abundance of 28Si, 29Si and 30Si is 92.23%, 4.67% and 3.10%, respectively. By the1920s, all three stable silicon isotopes had been discovered.
Worksheet_2_Answers.docx
If the atomic weight of silicon is 28.0855 and the natural abundance of Si-29 is 4.67%, what are the natural abundances of Si-28 and Si-30?
Periodic Table–Silicon – USGS — Isotope Tracers — Resources
Silicon has nine isotopes, with mass numbers from 25-33. … Si (the most abundant isotope, at 92.23%), 29Si (4.67%), and 30Si (3.1%) are stable; 32Si is a …
What is the mass number of silicon-29?
Silicon-29 atom is the stable isotope of silicon with relative atomic mass 28.9764947, 4.683 atom percent natural abundancy, and nuclear spin (1)/2.
Is Si-28 an isotope?
isotopes of silicon are known: silicon-28, which makes up 92.21 percent of the element in nature; silicon-29, 4.70 percent; and silicon-30, 3.09 percent. Five radioactive isotopes are known.
How To Find The Percent Abundance of Each Isotope – Chemistry
Images related to the topicHow To Find The Percent Abundance of Each Isotope – Chemistry
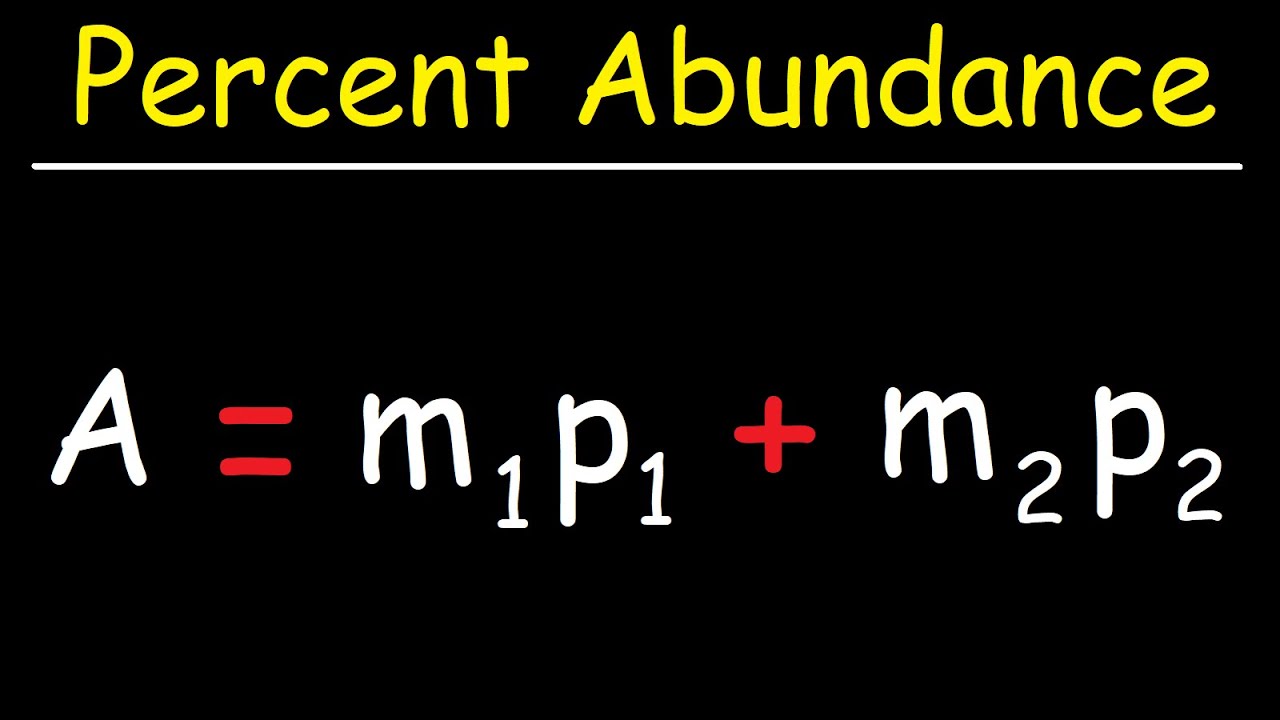
What is the mass of silicon 30?
silicon-30 atom (CHEBI:37976) The stable isotope of silicon with relative atomic mass 29.9737702. The least abundant (3.09 atom percent) isotope of naturally occurring silicon.
What is the element of s 32?
S-32 Information
Sulfur is a yellow, nonmetallic element belonging to group 16 of the periodic table. It is an essential element in living organisms, needed in the amino acids cysteine and methionine, and hence in many proteins. This element is absorbed by plants from the soil as sulphate ion.
How do you find the abundance of 3 isotopes?
- Step 1: Find the Average Atomic Mass. …
- Step 2: Set Up the Relative Abundance Problem. …
- Step 3: Solve for x to Get the Relative Abundance of the Unknown Isotope. …
- Step 4: Find percent abundance.
What is the percent abundance of an isotope?
Atoms that have the same number of protons but different numbers of neutrons are known as isotopes. Isotopes have different atomic masses. The relative abundance of an isotope is the percentage of atoms with a specific atomic mass found in a naturally occurring sample of an element.
What is the mass number of silicon?
How do you determine abundance?
Abundance is in simplest terms usually measured by identifying and counting every individual of every species in a given sector. It is common for the distribution of species to be skewed so that a few species take up the bulk of individuals collected.
How do you find the percent abundance of an isotope mass?
Step 1: List the known and unknown quantities and plan the problem. Change each percent abundance into decimal form by dividing by 100. Multiply this value by the atomic mass of that isotope. Add together for each isotope to get the average atomic mass.
What is the half-life of silicone?
When found in nature, silicon has three atomic mass numbers – 28, 29 and 30. Those three isotopes are considered highly stable in that they have no known half-life, which means they do not decay in any significant way.
How to Find the Abundance of Each Isotope
Images related to the topicHow to Find the Abundance of Each Isotope

Why is the atomic mass of silicon 28?
Silicon-28 atom is the stable isotope of silicon with relative atomic mass 27.9769265. The most abundant (92.23 atom percent) isotope of naturally occurring silicon. A trace element that constitutes about 27.6% of the earth’s crust in the form of SILICON DIOXIDE. It does not occur free in nature.
What is the average atomic mass of silicon 28 29 30?
There are three isotopes of silicon. They have mass numbers of 28, 29 and 30. The average atomic mass of silicon is 28.086amu.
Related searches to What is the natural abundance of silicon?
- what is the natural abundance of silicon isotopes
- what is the natural abundance of silicon atoms
- silicon 29 atomic mass
- who discovered silicon
- isotopes of silicon
- what is the natural abundance of silicon dioxide
- silicon isotopes natural abundance
- silicon 30
- uses of silicon
- percentage abundance of silicon 29
- relative atomic mass of silicon 28 29 30
- silicon-30
- what is the natural abundance of silicon on steel
- what is the natural abundance of silicon 28
Information related to the topic What is the natural abundance of silicon?
Here are the search results of the thread What is the natural abundance of silicon? from Bing. You can read more if you want.
You have just come across an article on the topic What is the natural abundance of silicon?. If you found this article useful, please share it. Thank you very much.