Are you looking for an answer to the topic “What is weight average atomic mass of silicon if it occurs naturally in 3 isotopes?“? We answer all your questions at the website Ecurrencythailand.com in category: +15 Marketing Blog Post Ideas And Topics For You. You will find the answer right below.
Multiply the amu by the percentage of occurrence to arrive at an average atomic mass of 28.0891. Was this answer helpful?‘Three isotopes of silicon occur in nature: 28Si with an atomic mass of 27.977amu, 29Si with an atomic mass of 28.975amu, and 30Si with an atomic mass of 29.974amu.To calculate the average atomic mass, multiply the fraction by the mass number for each isotope, then add them together. Whenever we do mass calculations involving elements or compounds (combinations of elements), we always use average atomic masses.
What is the weight average atomic mass of silicon if it occurs naturally in three isotopes?
‘Three isotopes of silicon occur in nature: 28Si with an atomic mass of 27.977amu, 29Si with an atomic mass of 28.975amu, and 30Si with an atomic mass of 29.974amu.
How do you find the average atomic mass of three isotopes?
To calculate the average atomic mass, multiply the fraction by the mass number for each isotope, then add them together. Whenever we do mass calculations involving elements or compounds (combinations of elements), we always use average atomic masses.
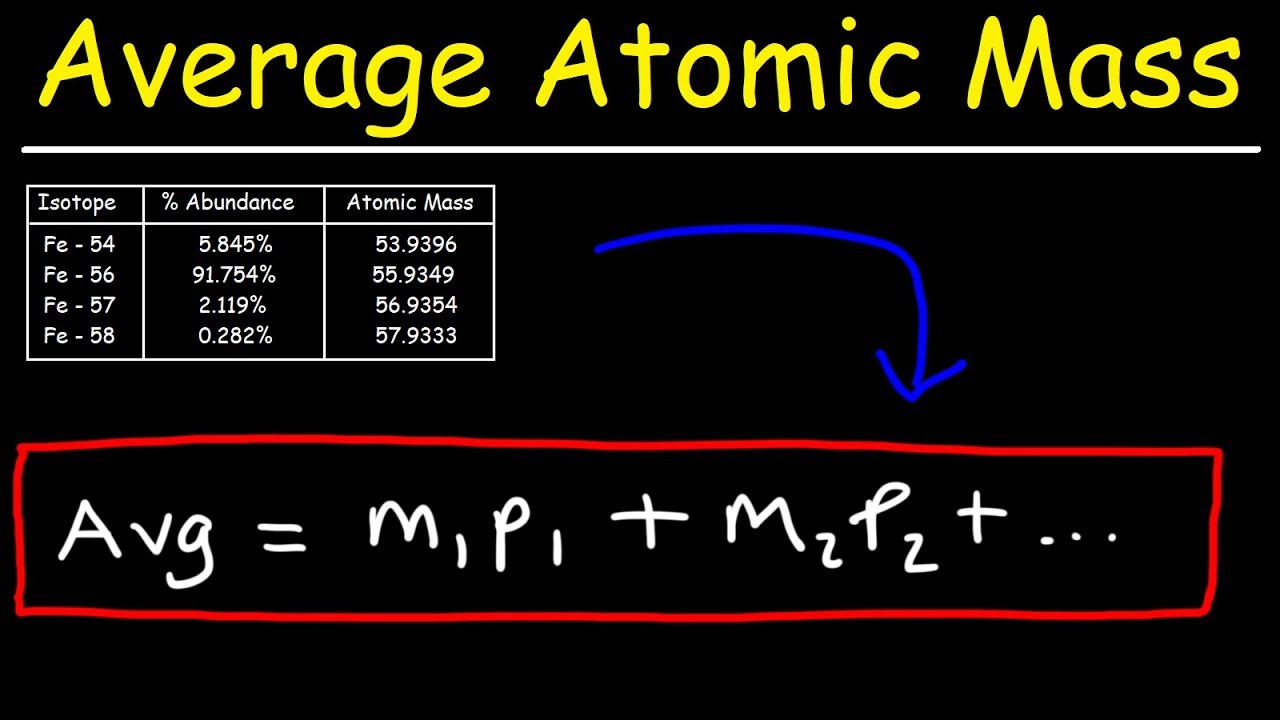
How To Calculate The Average Atomic Mass
Images related to the topicHow To Calculate The Average Atomic Mass

How do you calculate the average atomic mass of naturally occurring silicon?
Explanation: We take the amu of each isotope, multiply it by the percentage of occurrence, and end up with a weighted average: 27.9769×. 9218+28.9765×.
What is the weight average atomic mass of silicon?
What is the average atomic mass of silicon 28 29 30?
There are three isotopes of silicon. They have mass numbers of 28, 29 and 30. The average atomic mass of silicon is 28.086amu.
What is the atomic mass of silicon 28?
| ChEBI Name | silicon-28 atom |
|---|---|
| Definition | The stable isotope of silicon with relative atomic mass 27.9769265. The most abundant (92.23 atom percent) isotope of naturally occurring silicon. |
| Stars | This entity has been manually annotated by the ChEBI Team. |
| Supplier Information | |
| Download | Molfile XML SDF |
How do you find atomic mass from natural mass and isotope abundance?
Step 1: List the known and unknown quantities and plan the problem. Change each percent abundance into decimal form by dividing by 100. Multiply this value by the atomic mass of that isotope. Add together for each isotope to get the average atomic mass.
See some more details on the topic What is weight average atomic mass of silicon if it occurs naturally in 3 isotopes? here:
2.3: Isotope Abundance and Atomic Weight – Chemistry …
Answer: The correct answer is 36.9 amu. After watching the following two YouTubes, …
How many naturally occurring isotopes of silicon exist?
There are four isotopes of silicon (Si) exist in the natural environment—28Si, 29Si, 30Si and 32Si. The first three isotopes are stable isotopes and the last one is radiogenic. The relative abundance of 28Si, 29Si and 30Si is 92.23%, 4.67% and 3.10%, respectively.
What are the symbols of the three isotopes of silicon?
…
4.3Related Element.
| Element Name | Silicon |
|---|---|
| Element Symbol | Si |
| Atomic Number | 14 |
Physical Structure of Atoms: Calculate the Atomic Mass of Naturally Occurring Silicon
Images related to the topicPhysical Structure of Atoms: Calculate the Atomic Mass of Naturally Occurring Silicon

What is the atomic mass of silicon 29?
| ChEBI Name | silicon-29 atom |
|---|---|
| ChEBI ID | CHEBI:37974 |
| Definition | The stable isotope of silicon with relative atomic mass 28.9764947, 4.683 atom percent natural abundancy, and nuclear spin 1/2. |
| Stars | This entity has been manually annotated by the ChEBI Team. |
What is the average atomic mass unit of a single atom of silicon?
The mass of a single silicon atom in grams is 4.664 X 10−23 . The atomic mass of a silicon atom is 28.085 AMUs, which stands for atomic…
What is the atomic mass of silicon 30?
silicon-30 atom (CHEBI:37976) The stable isotope of silicon with relative atomic mass 29.9737702.
How do you find the abundance of 3 isotopes?
- Step 1: Find the Average Atomic Mass. …
- Step 2: Set Up the Relative Abundance Problem. …
- Step 3: Solve for x to Get the Relative Abundance of the Unknown Isotope. …
- Step 4: Find percent abundance.
What is the abundance of silicon 28 29 30?
The abundance of Si-28 is 92.23%. Si-29 is 4.68% and Si-30 is 3.09%. Because most Si atoms have a mass of 28 amu, the average mass of all silicon atoms is very close to 28.
What is the isotopes of silicon?
Periodic Table–Silicon. Silicon has nine isotopes, with mass numbers from 25-33. Si (the most abundant isotope, at 92.23%), 29Si (4.67%), and 30Si (3.1%) are stable; 32Si is a radioactive isotope produced by argon decay.
How do you calculate isotopic abundance?
To calculate the percent abundance of each isotope in a sample of an element, chemists usually divide the number of atoms of a particular isotope by the total number of atoms of all isotopes of that element and then multiply the result by 100.
Average Atomic Mass Practice Problems
Images related to the topicAverage Atomic Mass Practice Problems

How can I calculate weight?
It depends on the object’s mass and the acceleration due to gravity, which is 9.8 m/s2 on Earth. The formula for calculating weight is F = m × 9.8 m/s2, where F is the object’s weight in Newtons (N) and m is the object’s mass in kilograms.
Is atomic weight and atomic mass same?
Atomic weight, also called relative atomic mass, is the ratio of the total weight of the atoms of a chemical substance to a norm. The normal unit of atomic mass has been one-twelfth of the atomic mass of the carbon-12 isotope since 1961.
Related searches to What is weight average atomic mass of silicon if it occurs naturally in 3 isotopes?
- relative atomic mass of silicon 28 29 30 brainly
- atomic mass of silicon 30
- what is the atomic mass of silicon
- atomic mass of carbon
- calculate the average atomic mass for the two isotopes of rubidium
- atomic mass of silicon 28
- calculate the atomic mass of silicon the three silicon isotopes
- naturally occurring silicon consists of three isotopes
- calculate the atomic mass of silicon. the three silicon isotopes
Information related to the topic What is weight average atomic mass of silicon if it occurs naturally in 3 isotopes?
Here are the search results of the thread What is weight average atomic mass of silicon if it occurs naturally in 3 isotopes? from Bing. You can read more if you want.
You have just come across an article on the topic What is weight average atomic mass of silicon if it occurs naturally in 3 isotopes?. If you found this article useful, please share it. Thank you very much.