Are you looking for an answer to the topic “What will be the final temperature of a system in which 150.0 g of water at 5.0 C are added to 1.00 L of water at 90.5 C?“? We answer all your questions at the website Ecurrencythailand.com in category: +15 Marketing Blog Post Ideas And Topics For You. You will find the answer right below.
The final temperature is 79.4 degrees Celsius.The final temperature of the water will be 31.2 °C.Add the change in temperature to your substance’s original temperature to find its final heat. For example, if your water was initially at 24 degrees Celsius, its final temperature would be: 24 + 6, or 30 degrees Celsius.
What is the final temperature when 20.0 g of water at 25 C is mixed with 30.0 g of water at 80 C in an insulated calorimeter?
The final temperature of the water will be 31.2 °C.
How do you find the final temperature of a system?
Add the change in temperature to your substance’s original temperature to find its final heat. For example, if your water was initially at 24 degrees Celsius, its final temperature would be: 24 + 6, or 30 degrees Celsius.
Final Temperature Calorimetry Practice Problems – Chemistry
Images related to the topicFinal Temperature Calorimetry Practice Problems – Chemistry

What is the final temperature of the water at thermal equilibrium?
When equilibrium is reached, the temperature of the water is 23.9 °C. (Assume a density of 0.998 g/mL for water.)
What is the final temperature when 42.3 grams of water?
What is the final temperature when 42.3 g of water at 0.0 °C and 255.8 grams of water at 76.78 °C are mixed? The technique covered in worksheet #1 is used and the answer the ChemTeam gets is 65.9 °C. The above is (ice melts) + (liquid temp up) = (steam condenses) + (liquid temp down).
What happens to the temperature of a solid block of ice at 0 C when energy is added?
Changing states of matter and energy
A block of ice is solid water. When heat (a form of energy) is added, the ice melts into liquid water. It has reached its melting point – 0°C. Continue to apply heat, and the water will turn into water vapour, which is water in its gaseous state.
What is the formula to calculate temperature?
The temperature change depends on the amount of heat released or absorbed. Temperature difference = amount of heat absorbed or released/ mass of the body* specific heat of the body. ΔT = Q/m*c.
What is the final temperature after 840 joules is absorbed by 10.0 g of water at 25.0 OC?
final temperature of water would be 45°C
also read similar questions: a bucket contains 8 kg of water at 25°c.
See some more details on the topic What will be the final temperature of a system in which 150.0 g of water at 5.0 C are added to 1.00 L of water at 90.5 C? here:
what will be the final temperature of a … – Jiskha Homework Help
heat absorbed by cool water + heat lost by warm water = 0 · 150.0g x 4.184x(85.5)= 53659.80 · You didn’t substitute correctly. · BTW, thanks for showing your work.
what will be the final temperature of a system in which 150.0g …
what will be the final temperature of a system in which 150.0g of water at 5.0 degrees C are added to 1.00L of water at 90.5 degrees C? 64,566 results. Science.
What Will Be The Final Temperature?
4 What will be the final temperature of a system in which 150.0 g of water at 5.0 C are added to 1.00 L of water at 90.5 C? 5 How …
Ch. 9 & 10 Flashcards | Quizlet
If you add 1.33 MJ of heat to 500 g of water at 50°C in a sealed container, what is the final temperature of the steam? The latent heat of vaporization of water …
What is the temperature formula?
| Unit | To Celsius | To Fahrenheit |
|---|---|---|
| Celsius (C) | C (°) | C(9⁄5) + 32 |
| Fahrenheit | (F − 32) × 5⁄9 | F |
| Kelvin | K – 273.15 | (K – 273.15) * 9/5 + 32 |
Calorimetry: Using q=mΔTc to find Temperature + Example
Images related to the topicCalorimetry: Using q=mΔTc to find Temperature + Example

How do you calculate the final temperature of a mixture of ice and steam?
During condensation, Q2=m(steam)L(heat of vaporization) is released. When the condensed steam cools down from 100 °C to the final temperature T, Q3=c(water)m(steam)(100-T) heat is released. q1=c(ice)m(ice)(0-(-40)) is the heat needed to warm up the ice to 0°C.
What is the equation for change in temperature ΔT calculated?
Subtract the final and initial temperature to get the change in temperature (ΔT). Multiply the change in temperature with the mass of the sample. Divide the heat supplied/energy with the product. The formula is C = Q / (ΔT ⨉ m) .
How do you find the temperature change of water?
This is easy. You subtract the final temperature from the starting temperature to find the difference. So if something starts at 50 degrees Celsius and finishes at 75 degrees C, then the change in temperature is 75 degrees C – 50 degrees C = 25 degrees C.
How do you find the final temperature of two objects?
Calculate the final temperature of the water mixture using the equation T(final) = (m1_T1 + m2_T2) / (m1 + m2), where m1 and m2 are the weights of the water in the first and second containers, T1 is the temperature of the water in the first container and T2 is the temperature of the water in the second container.
How do you calculate heat capacity?
To calculate heat capacity, use the formula: heat capacity = E / T, where E is the amount of heat energy supplied and T is the change in temperature. For example, if it takes 2,000 Joules of energy to heat up a block 5 degrees Celsius, the formula would look like: heat capacity = 2,000 Joules / 5 C.
What happens when ice reaches 0 C?
At temperatures below 32°F (0°C), liquid water freezes; 32°F (0°C) is the freezing point of water. At temperatures above 32°F (0°C), pure water ice melts and changes state from a solid to a liquid (water); 32°F (0°C) is the melting point.
What happens when you heat water at 0 C?
The temperature increases until the ice reaches its melting point. What happens when you heat ice that is at 0∘∘C? The temperature stays constant until all the ice is melted.
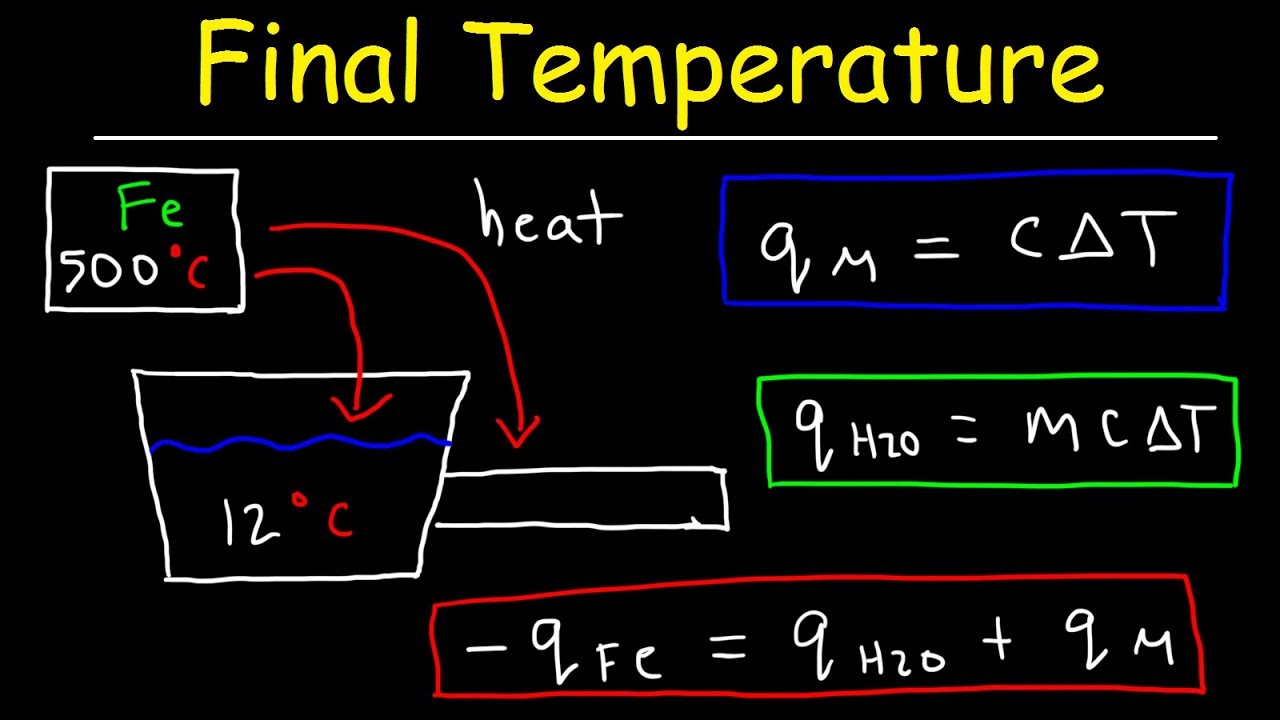
AP Specific Heat (Final Temp. Metal Dropped into Water)
Images related to the topicAP Specific Heat (Final Temp. Metal Dropped into Water)

When water cools from 10 C and then freezes to become ice Which of the following best describes describe the heat flow between the system and surroundings?
When water cools from 10 °C and then freezes to become ice, which of the following best describes describe the heat flow between the system and surroundings? The water is releasing energy as its temperature decreases, and that energy is absorbed by the surroundings.
How do you find final temperature given mass and initial temperature?
q=mc(Tf−Ti) , where: q is energy, m is mass, c is specific heat capacity, Tf is final temperature, and Ti is the initial temperature.
Related searches to What will be the final temperature of a system in which 150.0 g of water at 5.0 C are added to 1.00 L of water at 90.5 C?
- final temperature calculator
- how to find final temperature of a metal in water
Information related to the topic What will be the final temperature of a system in which 150.0 g of water at 5.0 C are added to 1.00 L of water at 90.5 C?
Here are the search results of the thread What will be the final temperature of a system in which 150.0 g of water at 5.0 C are added to 1.00 L of water at 90.5 C? from Bing. You can read more if you want.
You have just come across an article on the topic What will be the final temperature of a system in which 150.0 g of water at 5.0 C are added to 1.00 L of water at 90.5 C?. If you found this article useful, please share it. Thank you very much.