Are you looking for an answer to the topic “What will be the kinetic energy of photoelectrons ejected by a metal?“? We answer all your questions at the website Ecurrencythailand.com in category: +15 Marketing Blog Post Ideas And Topics For You. You will find the answer right below.
Chemistry. Q. The maximum kinetic energy of photoelectrons ejected from a metal, when it is irradiated with radiation of frequency 2×1014s−1 is 6.63×10−20J.The kinetic energy of an ejected electron equals the photon energy minus the binding energy of the electron in the specific material. An individual photon can give all of its energy to an electron. The photon’s energy is partly used to break the electron away from the material.The maximum kinetic energy of a photoelectron is given by 𝐸 = ℎ 𝑐 𝜆 − 𝑊 , m a x where ℎ is the Planck constant, 𝑐 is the speed of light, 𝜆 is the wavelength of the incident photon, and 𝑊 is the work function of the metal surface.

What is the kinetic energy of the ejected electron?
The kinetic energy of an ejected electron equals the photon energy minus the binding energy of the electron in the specific material. An individual photon can give all of its energy to an electron. The photon’s energy is partly used to break the electron away from the material.
What is the kinetic energy of the photoelectrons?
The maximum kinetic energy of a photoelectron is given by 𝐸 = ℎ 𝑐 𝜆 − 𝑊 , m a x where ℎ is the Planck constant, 𝑐 is the speed of light, 𝜆 is the wavelength of the incident photon, and 𝑊 is the work function of the metal surface.
The maximum kinetic energy of photoelectrons ejected from a metal, when it is irradiated
Images related to the topicThe maximum kinetic energy of photoelectrons ejected from a metal, when it is irradiated

Is kinetic energy of all photoelectrons from a given metal is same?
1 Answer. No. Depending upon the position and state of an electron in a metal when it absorbs an incident photon, a photoelectron can have kinetic energy ranging from 0 to a certain maximum value equal to the photon energy minus the work function of the metal.
What is the kinetic energy of a photoelectron emitted when radiation of frequency?
The maximum kinetic energy of the photo-electrons is found to be 6. 63×10−19J when the metal is irradiated with a radiation of frequency 3×1015Hz.
What is the kinetic energy formula?
Kinetic energy is directly proportional to the mass of the object and to the square of its velocity: K.E. = 1/2 m v2. If the mass has units of kilograms and the velocity of meters per second, the kinetic energy has units of kilograms-meters squared per second squared.
How do you find the kinetic energy of an electron?
Note that 1 eV is the kinetic energy acquired by an electron or a proton acted upon by a potential difference of 1 volt. The formula for energy in terms of charge and potential difference is E = QV. So 1 eV = (1.6 x 10^-19 coulombs)x(1 volt) = 1.6 x 10^-19 Joules.
What is the maximum kinetic energy of emitted photoelectrons?
The maximum kinetic energy of photoelectrons emitted from a surface when photons of energy 6 eV fall on it is 4 eV.
See some more details on the topic What will be the kinetic energy of photoelectrons ejected by a metal? here:
Photoelectric effect (article) | Photons | Khan Academy
The kinetic energy of emitted photoelectrons should increase with the light amplitude. The rate of electron emission, which is proportional to the measured …
Lesson Explainer: The Kinetic Energy of Photoelectrons
Thus, the maximum wavelength of incident light that will cause electrons to be emitted from the surface of the metal is 300 nm. Part 2. Recall that the formula …
Photoelectric Effect – University Physics Volume 3 – BC Open …
The maximum kinetic energy of a photoelectron at the metal surface is the difference between the energy of the incident photon and the work function of the …
What does kinetic energy of photoelectrons depend on?
The maximum kinetic energy of the emitted photoelectrons depends on the frequency of the incident light but is independent of its intensity.
Photoelectric Effect, Work Function, Threshold Frequency, Wavelength, Speed Kinetic Energy, Electr
Images related to the topicPhotoelectric Effect, Work Function, Threshold Frequency, Wavelength, Speed Kinetic Energy, Electr
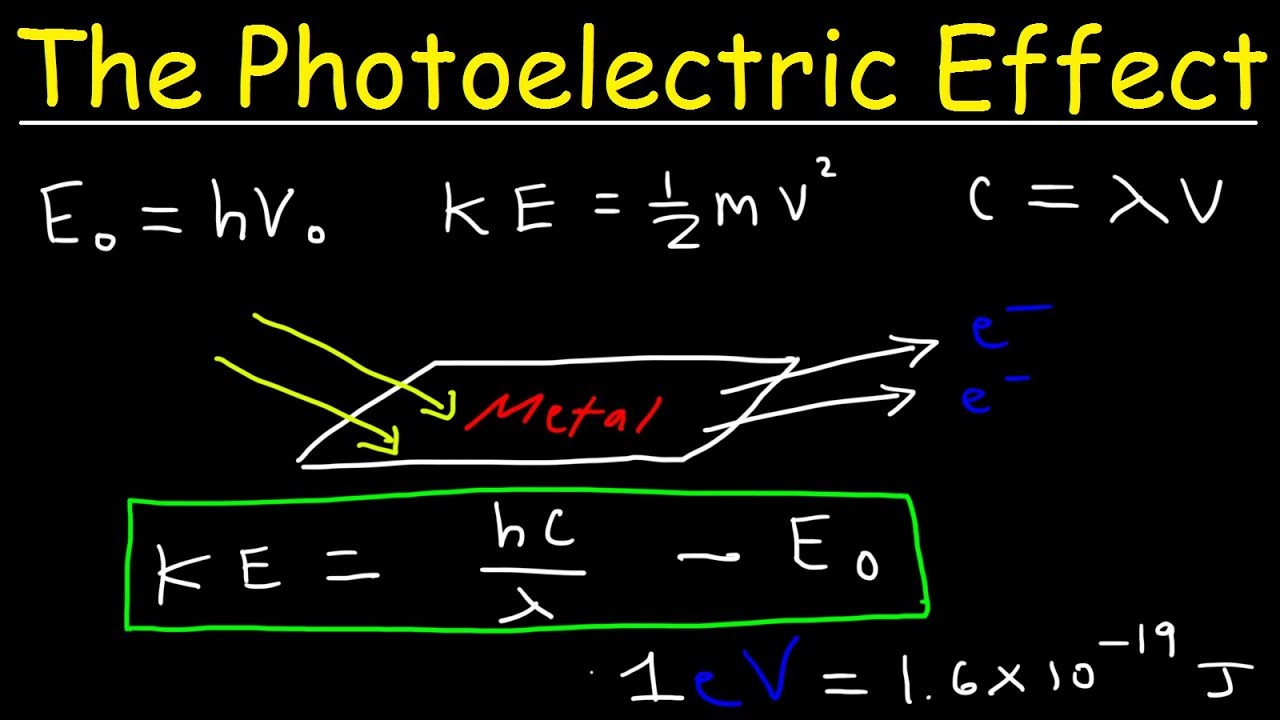
Why is maximum kinetic energy of photoelectrons?
The maximum kinetic energy of a photoelectron at the metal surface is the difference between the energy of the incident photon and the work function of the metal. The work function is the binding energy of electrons to the metal surface. Each metal has its own characteristic work function.
Do photoelectrons have same kinetic energy?
No. The energy of a photon is proportional to its frequency.
Do emitted electrons have same kinetic energy?
Solution : Kinetic energy of emitted electron depends on the frequency of incident radiation.
Do you expect all the ejected electrons to have the same kinetic energy?
No, it is not necessary that all the ejected electrons will have the same energy. Because the kinetic energy of the electron is the excess energy than…
What is kinetic energy class 11?
Kinetic energy is a form of energy which helps an object to remain in motion. When an object is applied net force, that object gains speed and eventually, gains kinetic energy. Kinetic theory is a chapter in class 11th physics.
What is kinetic energy in physics class 9?
Hint:Kinetic energy is the energy that an object possesses due to its motion. It is basically the energy of mass in motion. Kinetic energy can never be negative and it is a scalar quantity i.e. it provides only the magnitude and not the direction.
How do you calculate change in kinetic energy?
How do you calculate the change in kinetic energy? To calculate the amount of kinetic energy of an object, multiply its mass by 1/2, then multiply by the velocity squared.
Will electrons be ejected from the metal? (Photoelectric Effect Example)
Images related to the topicWill electrons be ejected from the metal? (Photoelectric Effect Example)

How do you find final kinetic energy?
Final kinetic energy KE = 1/2 m1v’12 + 1/2 m2v’22 = joules. For ordinary objects, the final kinetic energy will be less than the initial value. The only way you can get an increase in kinetic energy is if there is some kind of energy release triggered by the impact.
What will be the maximum kinetic energy of the photoelectrons if a certain metal is illuminated with a light of frequency?
When a metal is illuminated with light of frequency f, the maximum kinetic energy of the photoelectrons is 1.2 eV.
Related searches to What will be the kinetic energy of photoelectrons ejected by a metal?
- kinetic energy of ejected electron formula
Information related to the topic What will be the kinetic energy of photoelectrons ejected by a metal?
Here are the search results of the thread What will be the kinetic energy of photoelectrons ejected by a metal? from Bing. You can read more if you want.
You have just come across an article on the topic What will be the kinetic energy of photoelectrons ejected by a metal?. If you found this article useful, please share it. Thank you very much.