Are you looking for an answer to the topic “What will happen if dilute HCl is added to zinc granules Support your answer with a balanced chemical equation?“? We answer all your questions at the website Ecurrencythailand.com in category: +15 Marketing Blog Post Ideas And Topics For You. You will find the answer right below.
Keep Reading

What will happen if dilute HCl is added to zinc granules?
Answer: (d) A colourless and odourless gas is evolved.
A colourless and odourless gas is produced with bubbles when diluting hydrochloric acid is introduced to the granulated zinc placed in the test tube.
What happens when zinc reacts with dilute hydrochloric acid give balanced equation?
Zn+2HCl→ZnCl2+H2↑
Reaction Of Zinc with Hydrochloric acid | Chemistry demonstration
Images related to the topicReaction Of Zinc with Hydrochloric acid | Chemistry demonstration

When dilute HCl is added to a test tube containing zinc granules which of the following is not observed?
Hydrogen is evolved by the reaction of dilute non oxidizing acids on reactive metal such as zinc and burns with a pop sound. The solution remained colourless.
What happens when zinc granules react with dilute hydrochloric acid or Sulphuric acid?
When dilute sulphuric acid is poured on zinc granules, then zinc being more reactive than hydrogen displaces it from the acid and forms zinc sulphate and hydrogen gas.
What happens when zinc reacts with dilute HCl Class 8?
hydrochloric acid to form zinc chloride and hydrogen gas.
When dilute hydrochloric acid reacts with zinc metal Which of the following is needed for the identification of excreted gas?
The pH paper will be required to identify the gas evolved when dilute hydrochloric acid reacts with zinc metal. Hence the correct answer is option D. The pH indicator can be termed as a halochromic chemical that is added in very less amounts to any solution so that the pH of solution is visually determined.
When a student added zinc granules to dilute HCI a Colourless and Odourless gas was evolved which was tested with a burning matchstick It was observed that?
When the colourless gas was tested with a burning matchstick the matchstick extinguished and the gas burnt with a pop sound. This is because when zinc granules are added to dilute HCl hydrogen gas is evolved.
See some more details on the topic What will happen if dilute HCl is added to zinc granules Support your answer with a balanced chemical equation? here:
Write the chemical equation representing the reaction of zinc …
Zinc reacts with dilute HCl to form zinc chloride and hydrogen gas. Zn+2HCl→ZnCl2+H2↑ · A metal X forms a water-soluble salt XNO3. · The given equation …
what happens when dilute hydrochloric acid is poured on a …
Zinc reacts with dilute HCl to give a metallic zinc chloride with the evolution of hydrogen gas. Zn + HCl —> ZnCl2 + H2. Iron reacts with …
2.18: Recognizing Chemical Reactions – Chemistry LibreTexts
When zinc reacts with hydrochloric acid, the reaction bubbles vigorously as hydrogen gas is produced. The production of a gas is also an …
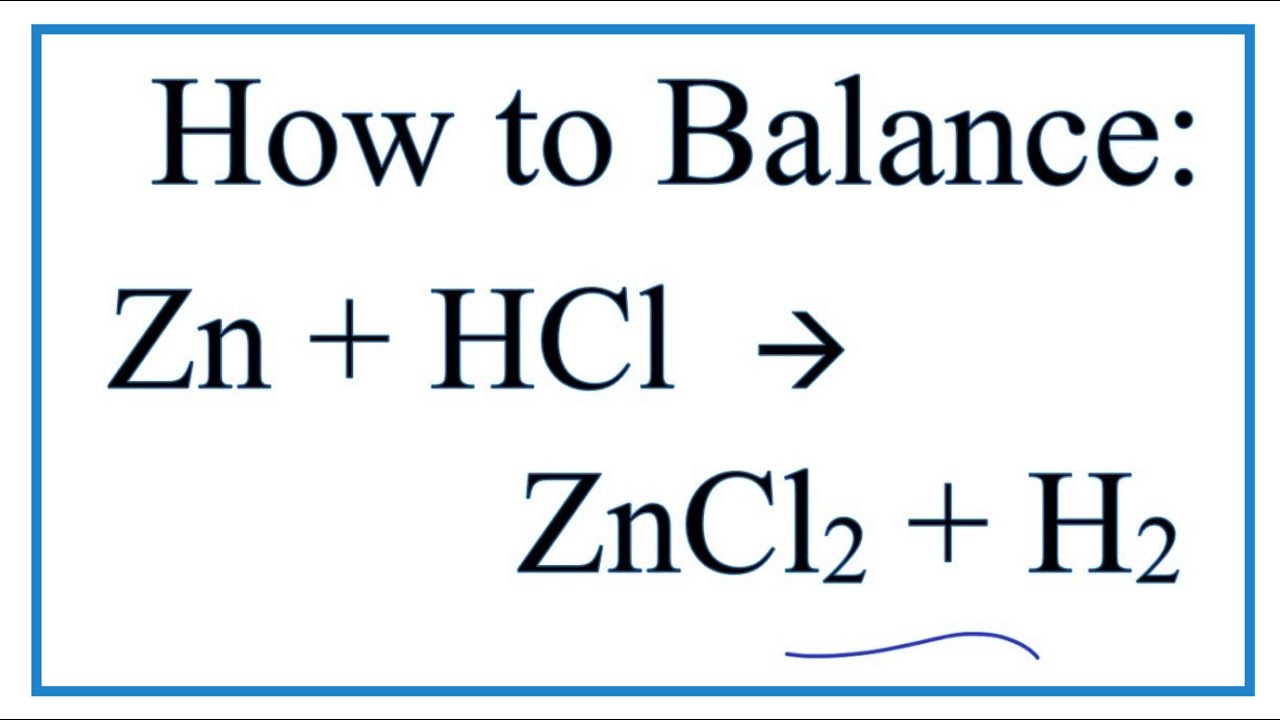
How to Balance Zn + HCl = ZnCl2 + H2
Images related to the topicHow to Balance Zn + HCl = ZnCl2 + H2
What did the student observe when he added dilute HCl to a test tube containing zinc granules?
Thus, when dilute hydrochloric acid is added to granulated zinc placed in a test tube, the observation made is a colourless and odourless gas evolves with bubbles. Thus, the correct option is (D) a colourless and odourless gas evolves with bubbles.
What happened to the colour of Zn granules after reaction with dil HCl?
The zinc chloride formed is white in colour. Thus, after the reaction of zinc and dilute HCl, the colour of zinc changes from grey to white because it forms a compound.
What happens to zinc granules when dilute hydrochloric acid or sulphuric acid is added to it is there any change in its temperature?
Zinc chloride is formed when Zn is combined with dilute sulphuric acid and hydrogen gas is liberated. The reaction is exothermic, and thus the reaction generates heat.
When zinc granules are added to dilute sulfuric acid hydrogen is given off and the metal dissolves?
Zinc melts at 420∘C . When zinc granules are added to dilute sulfuric acid, hydrogen is given off and the metal dissolves. Zinc has a hardness on the Mohs scale of 2.5 and a density of 7.13 g/cm3 at 25∘C It reacts slowly with oxygen gas at elevated temperatures to form zinc oxide, Zno.
When zinc granules react with dilute hydrochloric acid which element present in the acid is replaced by the metal?
The hydrochloric acid also donates the proton and accepts electrons from any metal on treatment with. This is an example of a single replacement reaction where zinc metal displaces the hydrogen to form zinc chloride and hydrogen gas.
Reaction of Zinc with Dilute Hydrochloric acid | Displacement reaction
Images related to the topicReaction of Zinc with Dilute Hydrochloric acid | Displacement reaction

What happens when Zn reacts with HCl?
When zinc reacts with hydrochloric acid it produces zinc chloride and hydrogen gas.
What will you observe when zinc granules are added to a dilute hydrochloric acid in a beaker write the chemical equation?
When dilute hydrochloric acid is added to granulated zinc placed in a test tube, zinc metal is converted to zinc chloride and hydrogen gas is evolved in the reaction. In the reaction we can see that a zinc chloride salt is formed and hydrogen gas is evolved. The evolved hydrogen gas is colorless and odorless.
Related searches to What will happen if dilute HCl is added to zinc granules Support your answer with a balanced chemical equation?
- when zinc metal is mixed with hydrochloric acid vigorous
- when zinc is immersed in hydrochloric acid produces hydrogen gas this is a physical change
- how do we test the gas product when zinc reacts with hcl
- what gas was produced by the reaction of zinc and hydrochloric acid
- zinc and dilute hydrochloric acid equation
- zinc and hydrochloric acid experiment
- reaction between zinc and hydrochloric acid observation
- zinc metal formula
Information related to the topic What will happen if dilute HCl is added to zinc granules Support your answer with a balanced chemical equation?
Here are the search results of the thread What will happen if dilute HCl is added to zinc granules Support your answer with a balanced chemical equation? from Bing. You can read more if you want.
You have just come across an article on the topic What will happen if dilute HCl is added to zinc granules Support your answer with a balanced chemical equation?. If you found this article useful, please share it. Thank you very much.