Are you looking for an answer to the topic “What would a scientist use a calorimeter for quizlet?“? We answer all your questions at the website Ecurrencythailand.com in category: +15 Marketing Blog Post Ideas And Topics For You. You will find the answer right below.
Keep Reading

What would a scientist use a calorimeter for?
A calorimeter is a device used to measure the heat flow of a chemical reaction or physical change. Energy may be defined as the ability to do work.
Why is using a calorimeter important?
Because calorimetry is used to measure the heat of a reaction, it is a crucial part of thermodynamics. In order to measure the heat of a reaction, the reaction must be isolated so that no heat is lost to the environment. This is achieved by use of a calorimeter, which insulates the reaction to better contain heat.
Calorimeter | Reactions | Chemistry | FuseSchool
Images related to the topicCalorimeter | Reactions | Chemistry | FuseSchool

What are two commonly used calorimeters?
Calorimeters are equipment used to measure the enthalpy, which is the heat energy, of a reaction. There are two commonly used types of calorimeters: coffee cup calorimeters and bomb calorimeters.
What is calorimetry in chemistry quizlet?
Calorimetry: The science of measuring heat flow that occurs during a physical or chemical change.
What is a calorimeter experiment?
In the laboratory, heat flow is measured in an apparatus called a calorimeter. A calorimeter is a device used to determine heat flow during a chemical or physical change.
How is calorimetry used in chemistry?
Calorimetry is used to measure amounts of heat transferred to or from a substance. To do so, the heat is exchanged with a calibrated object (calorimeter). The temperature change measured by the calorimeter is used to derive the amount of heat transferred by the process under study.
How is calorimetry used in real world applications?
Calorimeters are useful in various industries and academic settings, an industrial pilot plant can use a DSC to determine a change in a products formula and how it affects the formula itself. Oxygen bomb calorimeters are useful in food testing laboratories to determine the amount of heat (calories) in food.
See some more details on the topic What would a scientist use a calorimeter for quizlet? here:
Calorimetry Flashcards | Quizlet
A calorimeter is a device used to measure the heat flow of a chemical reaction or physical change.
Chemistry Calorimetry Flashcards | Quizlet
Calorimetry. The study of heat flow and heat measurement. These experiments determine the heat changes of reactions by changes in a calorimeter. ; Calorimeter.
CALORIMETRY Flashcards | Quizlet
calorimetry. the precise measurement of heat flow out of a system for chemical and physical processes ; calorimeter. an insulated device used to measure the …
Calorimetry Flashcards | Quizlet
Calorimetry: The science of measuring heat flow that occurs during a physical or chemical change ; Heat: Form of energy that moves from one object to another …
How is calorimetry used in medicine?
Pharmaceutical solution calorimetry is particularly useful during the development phase of a solid drug compound, as it allows researchers to perform analysis of crystalline content and verify stability.
CALORIMETRY_Part 01
Images related to the topicCALORIMETRY_Part 01
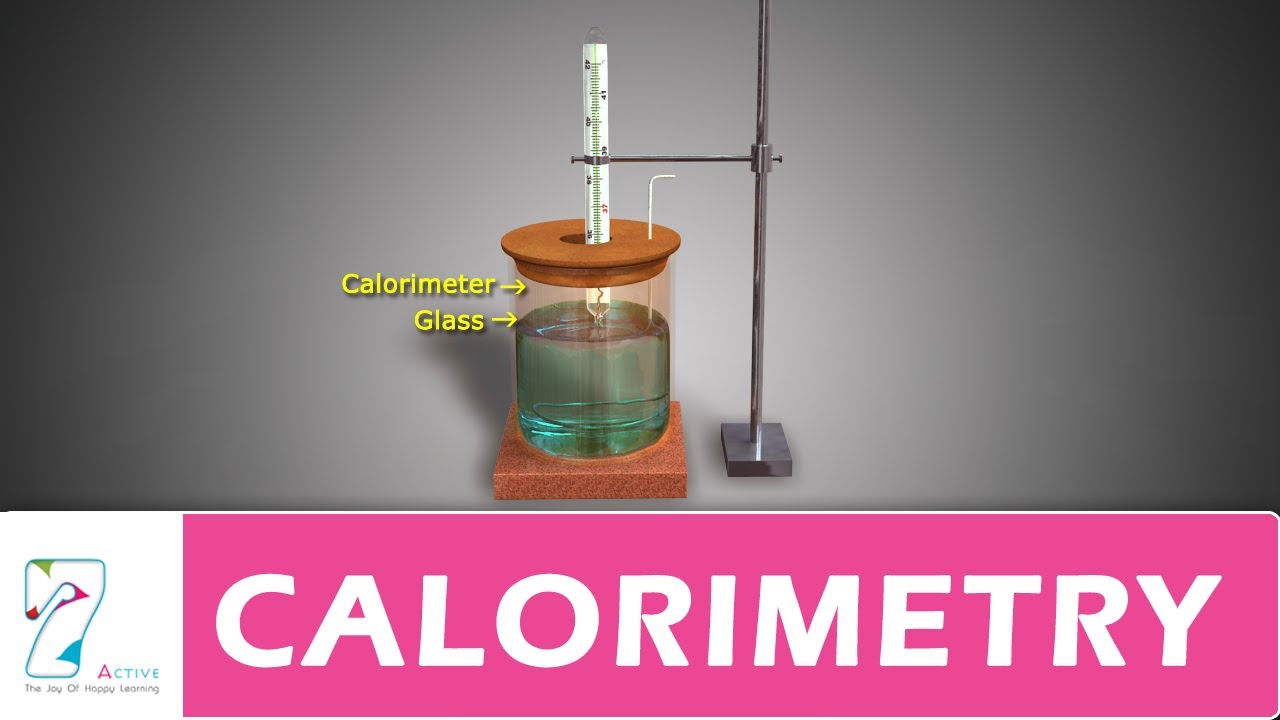
What is an example of a calorimeter?
The measurement of heat using a simple calorimeter, like the coffee cup calorimeter, is an example of constant-pressure calorimetry, since the pressure (atmospheric pressure) remains constant during the process. Constant-pressure calorimetry is used in determining the changes in enthalpy occurring in solution.
What is a simple calorimeter?
Simple calorimeters are made with a metal container of water, positioned above a combustion chamber. A thermometer is used to measure the heat change in the amount of water. The simplest versions of the device can be made at home using two coffee cups or styrofoam cups, though it is not as accurate as lab equipment.
What is a calorimeter in physics?
A calorimeter is a device used to measure the quantity of heat transferred to or from an object.
What does a bomb calorimeter measure quizlet?
A bomb calorimeter is an instrument that measures the heat energy released when food is burned, thus providing an estimate of the potential energy of the foods.
What is measured by the heat of reaction quizlet?
Describe enthalpy. Enthalpy, H, is a measure of the heat energy in a chemical system, and it cannot be measured.
What is the specific heat for water?
The units of specific heat are usually calories or joules per gram per Celsius degree. For example, the specific heat of water is 1 calorie (or 4.186 joules) per gram per Celsius degree.
What do scientists use to measure changes in thermal energy?
Thermal energy can be described as the amount of energy a body stored due to its being at a higher temperature than its surroundings . It is measured using something called a calorimeter.
Bomb Calorimetry
Images related to the topicBomb Calorimetry

What does a coffee cup calorimeter measure?
A coffee cup calorimeter is a constant pressure calorimeter. As such, the heat that is measured in such a device is equivalent to the change in enthalpy. A coffee cup calorimeter is typically used for solution based chemistry and as such generally involves a reaction with little or no volume change.
What is calorimetry for kids?
A calorimetry test measures your child’s resting metabolic rate. A resting metabolic rate shows how much energy (calories) the body burns when at rest. The body must burn energy at rest to keep up normal functions such as breathing and heart rate.
Related searches to What would a scientist use a calorimeter for quizlet?
- the specific heat s is the quantity of
- when heat is released by the system
- a calorimeter measures the heat involved in reactions or other processes by measuring the
- what would a scientist use a calorimeter for
- what is the general definition of heat capacity
- what is the general definition of heat capacity?
- select all of the statements below that correctly describe what is shown in the enthalpy diagram
- calorimetry using a bomb calorimeter quizlet
- how does the specific heat of a substance relate to its ability to change temperature
- identify the necessary parts to build a calorimeter
Information related to the topic What would a scientist use a calorimeter for quizlet?
Here are the search results of the thread What would a scientist use a calorimeter for quizlet? from Bing. You can read more if you want.
You have just come across an article on the topic What would a scientist use a calorimeter for quizlet?. If you found this article useful, please share it. Thank you very much.