Are you looking for an answer to the topic “When a chlorine atom forms an ion its radius?“? We answer all your questions at the website Ecurrencythailand.com in category: +15 Marketing Blog Post Ideas And Topics For You. You will find the answer right below.
11. When a chlorine atom forms an ion its radius increases, but when a sodium atom forms an ion its radius decreases.Answer: When a chlorine atom forms an ion, it gains electrons, making it negative. A neutral chlorine will become a chlorine with a -1 charge. When it gains an electron, the radius increases.What occurs when an atom of chlorine forms a chloride ion? (1) The chlorine atom gains an electron, and its radius becomes smaller. The chlorine atom gains an electron, and its radius becomes larger.

Table of Contents
When a chlorine atom forms an ion its radius increases Why?
Answer: When a chlorine atom forms an ion, it gains electrons, making it negative. A neutral chlorine will become a chlorine with a -1 charge. When it gains an electron, the radius increases.
When a chlorine atom becomes a chlorine ion its radius?
What occurs when an atom of chlorine forms a chloride ion? (1) The chlorine atom gains an electron, and its radius becomes smaller. The chlorine atom gains an electron, and its radius becomes larger.
Ionic and Atomic Radius – Periodic Trends
Images related to the topicIonic and Atomic Radius – Periodic Trends

When a chlorine atom forms an ion It has a?
Chlorine gains an electron, leaving it with 17 protons and 18 electrons. Since it has 1 more electron than protons, chlorine has a charge of −1, making it a negative ion. When ions form, atoms gain or lose electrons until their outer energy level is full.
Does chlorine atom or chlorine ion have a larger radius?
Hence, we can deduce that the forces of attraction exerted on the outermost electron in chloride ion is lesser than chlorine atom, since there are more electrons. Conclusion, chloride ion has a bigger atomic radius due to an increase in number of electrons.
Why does atomic radius decrease across a period?
Across a period, effective nuclear charge increases as electron shielding remains constant. A higher effective nuclear charge causes greater attractions to the electrons, pulling the electron cloud closer to the nucleus which results in a smaller atomic radius.
Why is the ionic radius of a chloride ion larger than the ionic radius of a sodium ion?
Why is the ionic radius of a chloride ion larger than the ionic radius of a sodium ion? (A) A chloride ion has one more occupied electron shell than a sodium ion.
What is ionic radius trend?
The ionic radius trend refers to how the ionic radius of elements follows a predictable trend across the periodic table of the elements. Ionic radius tends to increase as you move from top to bottom down the periodic table, and it tends to decrease as you move left to right across the periodic table.
See some more details on the topic When a chlorine atom forms an ion its radius? here:
When a chlorine atom forms an ion its radius increases, but …
Answer: When a chlorine atom forms an ion, it gains electrons, making it negative. A neutral chlorine will become a chlorine with a -1 …
12. When a chlorine atom forms an ion its radius increases …
Explanation: When a chlorine atom forms an ion, it gains electrons, making it negative. A neutral chlorine will become a chlorine with a -1 …
What happens when a chlorine atom becomes a chloride ion?
When Chlorine Cl gains an electron it forms the negative ion called Chlorine ion Cl- . Remember that when an atom gains an electron it forms an …
atomic and ionic radius – Chemguide
Negative ions are bigger than the atoms they come from. Chlorine is 2,8,7; Cl- is 2,8,8. Although the electrons are still all in the 3-level …
When an atom becomes a positive ion the radius of the atom?
Therefore, when an atom becomes a positive ion is pulls its electrons closer, decreasing is radius moreover, when it becomes a negative ion, it pulls its electrons closer and decreases the radius.
Why the chlorine atom has a smaller atomic radius than the sulfur atom?
Sulfur and chlorine are in the lowest period, so they have the largest atomic radii. Because sulfur is to the left of chlorine on the periodic table, it will have a larger atomic radius. As a result, sulfur has the largest atomic radius out of the possible options.
What will be the ion charge when chlorine forms an ion?
The chlorine atom, which has a high electronegativity, gains an electron and is converted into a chloride ion that has the same electron configuration as argon (1s2 2s22p6 3s23p6). The chloride ion has a − 1 charge because there are 17 protons in the nucleus, but there are 18 electrons around the nucleus of the ion.
What type of ion will chlorine tend to form?
Again, it is more energy-efficient for chlorine to gain one electron than to lose seven. Therefore, it tends to gain an electron to create an ion with 17 protons, 17 neutrons, and 18 electrons, giving it a net negative (–1) charge. It is now referred to as a chloride ion.
How many ions are in Cl?
There are two chloride ions in the formula. Although chlorine as an element is a diatomic molecule, Cl2, elemental chlorine is not part of this ionic compound. The chlorine is in the form of a negatively charged ion, not the neutral element.
Atoms form ions (Chemistry) – Binogi
Images related to the topicAtoms form ions (Chemistry) – Binogi
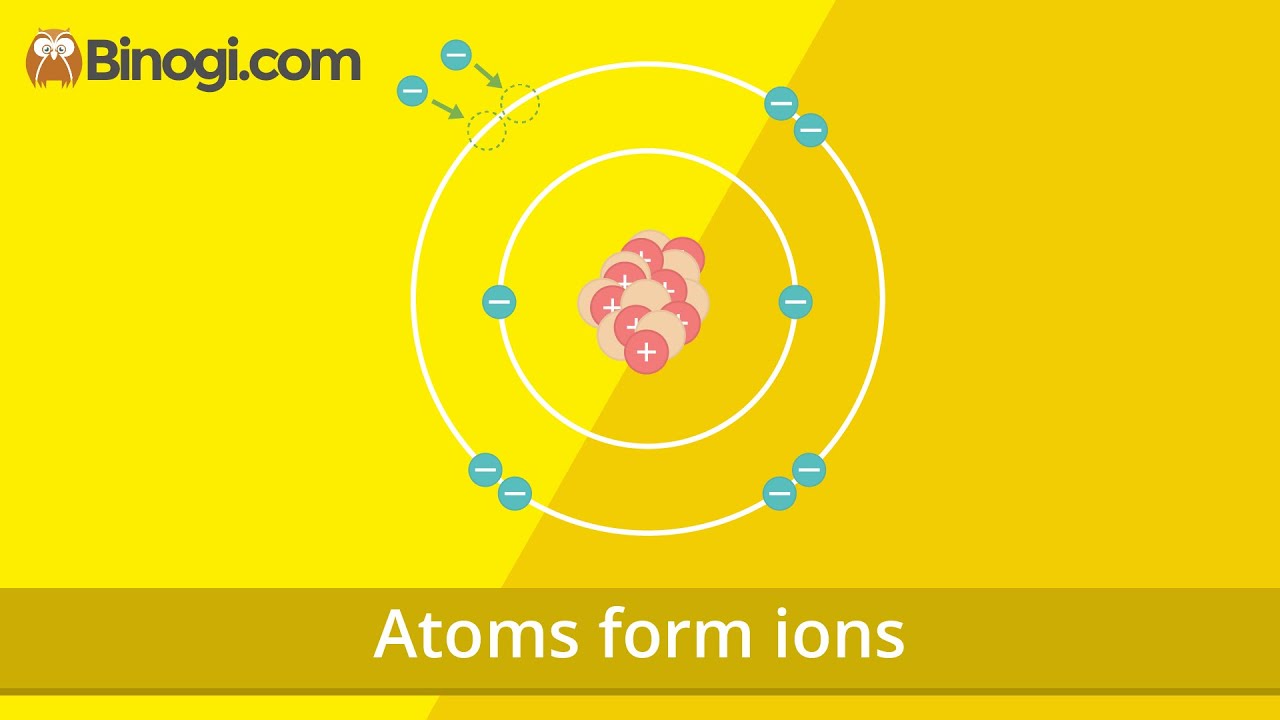
What is the radius of chlorine?
Would you expect a Cl-ion to be larger or smaller than an Mg2+ ion?
Would you expect a Cl- ion to be larger or smaller than a Mg2+ ion? Cl- would be larger because there are more electrons which would repel causing the atom to be larger.
Which ion has the largest radius?
Explanation: The ionic radii of cations follow the same trends as atomic radii. They increase from top to bottom and from right to left in the Periodic Table. Thus, the ion with the largest radius is closest to the lower left corner of the Periodic Table, and that is the K+ ion.
Does ionic radius increase across a period?
The size of an element’s ionic radius follows a predictable trend on the periodic table. As you move down a column or group, the ionic radius increases. This is because each row adds a new electron shell. Ionic radius decreases moving from left to right across a row or period.
What makes atomic radius increase?
An atom gets larger as the number of electronic shells increase; therefore the radius of atoms increases as you go down a certain group in the periodic table of elements. In general, the size of an atom will decrease as you move from left to the right of a certain period.
Why does atomic radius increases down the group?
In general, the atomic radius decreases and increases in a group over a time. The number of energy levels (n) increases in a group downwards, since there is a larger distance between the nucleus and the outermost orbital. This results in an atomic radius that is greater.
Is chlorine ion bigger than sodium ion?
Sodium atoms are much larger than chlorine atoms, but sodium ions are much smaller than chloride ions.
Why are chlorine ions bigger than sodium ions?
When you’re comparing ions, it’s different as opposed to comparing the elements themselves. The periodic table trends refer to the neutral elements, so naturally, Cl would be smaller than Na because the increasing protons pull in the electrons tighter.
Why are sodium ions smaller than chlorine ions?
So the sodium ion is actually going to be smaller. Because it gets rid of the electron in that third shell, and the chlorine cation, gained an electron, so it has completely completed its third shell.
What does ionic radius depend on?
Atomic and Ionic Radii
The size of an atom or ion depends on the size of the nucleus and the number of electrons. Generally atoms with higher numbers of electrons have larger radii than those with smaller numbers of electrons.
Cl- Electron Configuration (Chloride Ion)
Images related to the topicCl- Electron Configuration (Chloride Ion)

What affects ionic radius?
Ionic size (for the same ion) also increases with increasing coordination number, and an ion in a high-spin state will be larger than the same ion in a low-spin state. In general, ionic radius decreases with increasing positive charge and increases with increasing negative charge.
What represents increasing ionic radius?
When a negative ion is formed, one or more electrons are added to an atom and the effective nuclear charge is reduced. The electron cloud expands and the size of negative ion becomes more. Hence, the correct order of increasing ionic radii is Mg2+<Na+<F−<O2−.
Related searches to When a chlorine atom forms an ion its radius?
- choose the atom in each pair that has the largest atomic radius
- are ions of alkali metals larger or smaller than ions of alkaline earth metals from the same period
- for each of the following, circle the correct element
- when a sodium atom forms an ion its radius decreases
- which of the transition metals has the largest ionization energy
- what is the charge formed when an f atom forms an ion
- for each of the following circle the correct element
- when a chlorine atom forms an ion its radius increases
- circle the element in the following pairs has the greater ionization energy
- rank the following elements by increasing electronegativity: sulfur, oxygen, neon, aluminum
- what happens when a calcium atom forms an ion with a 2+ charge
- are metal ions larger or smaller than the neutral atoms they came from
- rank the following elements by increasing electronegativity sulfur oxygen neon aluminum
- when an atom forms ions
- how does a chlorine atom become an ion
Information related to the topic When a chlorine atom forms an ion its radius?
Here are the search results of the thread When a chlorine atom forms an ion its radius? from Bing. You can read more if you want.
You have just come across an article on the topic When a chlorine atom forms an ion its radius?. If you found this article useful, please share it. Thank you very much.
