Are you looking for an answer to the topic “When a sodium atom reacts with a chlorine atom to form a compound the electron configuration?“? We answer all your questions at the website Ecurrencythailand.com in category: +15 Marketing Blog Post Ideas And Topics For You. You will find the answer right below.
Explanation: During this reaction, the sodium loses one electron on its outer shell which then gives it the electron configuration of neon (2-8) and the chlorine gains one electron on its outer shell which then gives it the electron configuration of argon (2-8-8).A sodium atom loses an electron to a chlorine atom. The sodium atom becomes a positive sodium ion. The chlorine atom becomes a negative chloride ion. Both sodium ions and chloride ions have full electron shells.An ionic bond forms when the metal sodium gives up an electron to the nonmetal chlorine. In row 1 of Figure above, an atom of sodium donates an electron to an atom of chlorine (Cl). By losing an electron, the sodium atom becomes a sodium ion. It now has one less electron than protons, giving it a charge of +1.
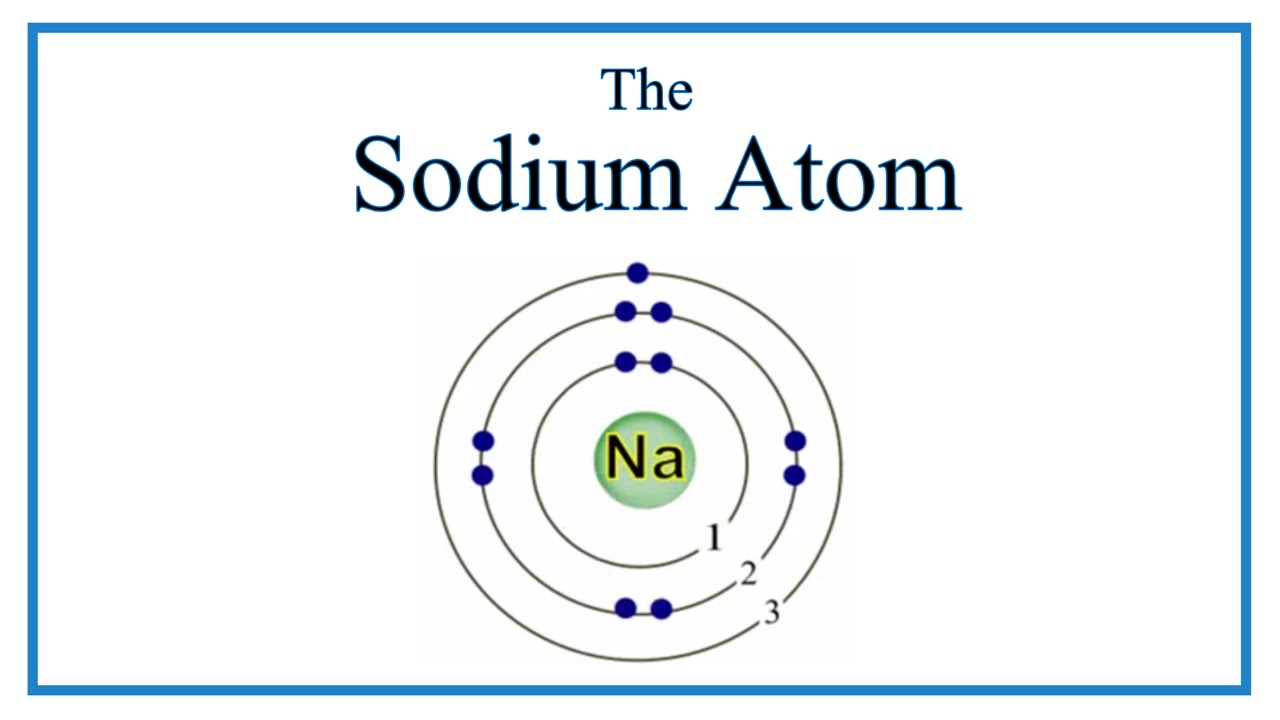
Table of Contents
What happens when a sodium atom reacts with a chlorine atom?
A sodium atom loses an electron to a chlorine atom. The sodium atom becomes a positive sodium ion. The chlorine atom becomes a negative chloride ion. Both sodium ions and chloride ions have full electron shells.
When a sodium atom gives up an electron to a chlorine atom it forms the type of bond called?
An ionic bond forms when the metal sodium gives up an electron to the nonmetal chlorine. In row 1 of Figure above, an atom of sodium donates an electron to an atom of chlorine (Cl). By losing an electron, the sodium atom becomes a sodium ion. It now has one less electron than protons, giving it a charge of +1.
Atomic Structure of the Sodium Atom (Na)
Images related to the topicAtomic Structure of the Sodium Atom (Na)

When sodium and chlorine combine to produce the compound NaCl the ions formed have the same electron configuration as atoms of?
In forming an ionic bond, the sodium atom, which is electropositive, loses its valence electron to chlorine. The resulting sodium ion has the same electron configuration as neon (1s2 2s22p6).
Which electron configuration is correct for a sodium ion?
What type of reaction is sodium and chlorine?
A reaction of sodium with chlorine to produce sodium chloride is an example of a combination reaction.
When an atom of chlorine forms an ionic bond with an atom of sodium the atom of chlorine?
When an atom of chlorine forms an ionic bond with an atom of sodium, the atom of chlorine gains a electron to form an anion. Explanation: An ionic bond is formed when an element completely transfers its valence electron to another element.
How many electrons will chlorine gain or lose when it forms an ion?
Chlorine will gain one electron when it forms an ion to be an anion with a charge of -1.
See some more details on the topic When a sodium atom reacts with a chlorine atom to form a compound the electron configuration? here:
Demonstrations – Sodium + Chlorine – Angelo State University
When a sodium atom transfers an electron to a chlorine atom, forming a sodium cation (Na+) and a chloride anion (Cl-), both ions have complete valence …
Ionic Bond – an overview | ScienceDirect Topics
In forming an ionic bond, the sodium atom, which is electropositive, loses its valence electron to chlorine. The resulting sodium ion has the same electron …
4.3: The Reaction of Sodium with Chlorine – Chemistry …
Neutral atoms and their associated ions have very different physical and chemical properties. Sodium atoms form sodium metal, a soft, …
How does sodium react with chlorine? | 14-16 years – RSC …
Answers · A sodium atom has one electron in the outer shell. · A chlorine atom seven electrons in the outer shell. · A sodium atom loses an electron to a chlorine …
What causes a polar bond?
A polar bond is a type of covalent bond. A bond between two or more atoms is polar if the atoms have significantly different electronegativities (>0.4). Polar bonds do not share electrons equally, meaning the negative charge from the electrons is not evenly distributed in the molecule. This causes a dipole moment.
How does covalent bonding take place?
A covalent bond consists of the mutual sharing of one or more pairs of electrons between two atoms. These electrons are simultaneously attracted by the two atomic nuclei. A covalent bond forms when the difference between the electronegativities of two atoms is too small for an electron transfer to occur to form ions.
How is ionic compound sodium chloride forms from sodium and chlorine?
When sodium reacts with chlorine, it transfers its one outermost electron to the chlorine atom. By losing one electron, sodium atom forms a sodium ion (Na+) and by gaining one electron, the chlorine atom forms a chloride ion (Cl-). Sodium ion has positive charge whereas chloride ions have negative charge.
Which chemical equation correctly represents the formation of an ionic bond between sodium and chlorine?
2Na (s) + Cl2 (g) 2NaCl (s)
This can be understood by saying that two sodium atoms combine with one diatomic chlorine molecule to form two ionic units of sodium chloride.
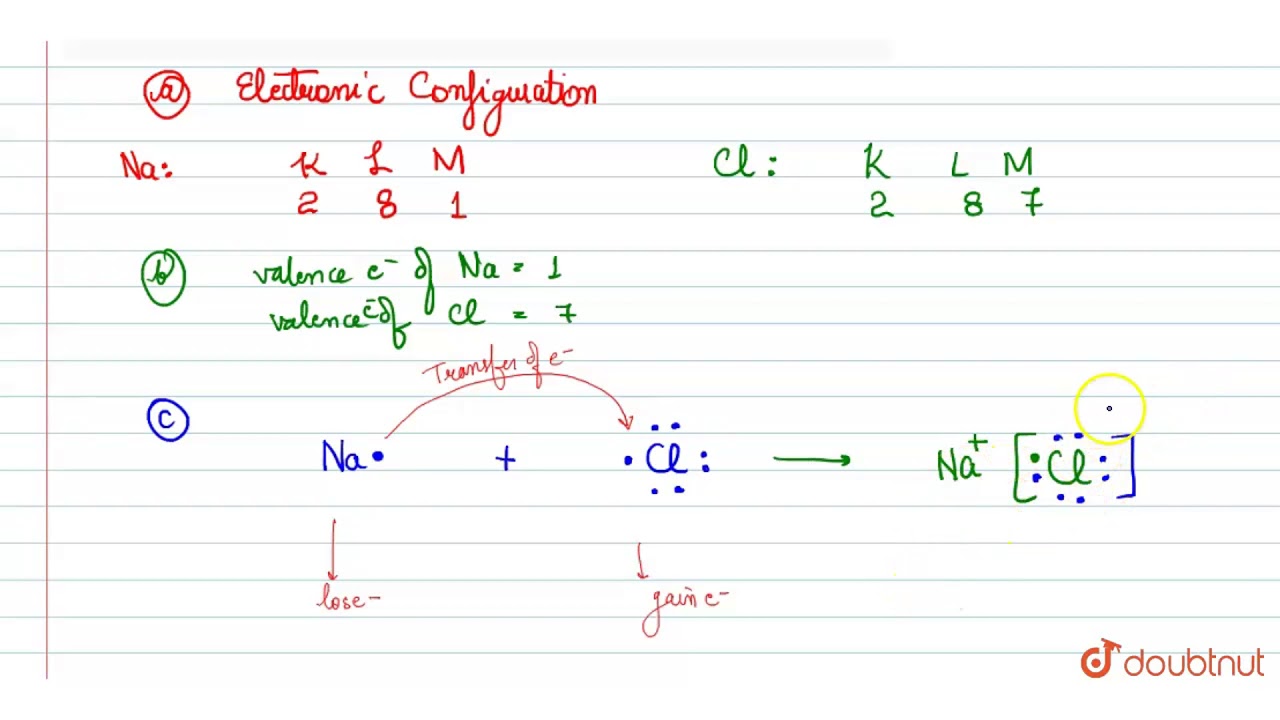
(a). Write down the electronic configuration of (i) Sodium atom, and (ii) Chlorine atom. (b). .
Images related to the topic(a). Write down the electronic configuration of (i) Sodium atom, and (ii) Chlorine atom. (b). .
How is NaCl ionic bond formed?
Sodium chloride (NaCl) is a typical ionic compound. The picture below shows both a sodium and a chlorine ion. Sodium has 1 electron in its outermost shell, and chlorine has 7 electrons. It is easiest for sodium to lose its electron and form a +1 ion, and for chlorine to gain an electron, forming a -1 ion.
What is the electron configuration of sodium chloride?
What is the electron configuration of chlorine?
What is the Aufbau configuration of sodium?
The Aufbau electron configuration method is 1s 2s 2p 3s 3p 4s 3d 4p 5s 4d 5p 6s 4f 5d 6p 7s 5f 6d. The first two electrons of sodium enter the 1s orbital and the next two electrons enter the 2s orbital. The p-orbital can have a maximum of six electrons.
What is the product when sodium reacts with chlorine?
The vigorous reaction between the elements sodium and chlorine forms the white, crystalline compound sodium chloride, common table salt, which contains sodium cations and chloride anions (Figure 4.3. 1).
What type of chemical equation is the following 2NaCl → 2Na Cl2?
The reaction 2Na + Cl2 = 2NaCl is an example of Combination reaction. Decomposition reaction will occur when one reactant breaks down into two or more products, e.g. AB => A + B. In Combination Reaction, two or more elements/compounds combine to form a single compound, e.g. A + B => AB.
What happens when a sodium and chlorine interact to form the salt sodium chloride?
When a sodium atom transfers an electron to a chlorine atom, forming a sodium cation (Na+) and a chloride anion (Cl–), both ions have complete valence shells, and are energetically more stable. The reaction is extremely exothermic, producing a bright yellow light and a great deal of heat energy.
What is the ionic charge of chlorine when it forms an ionic bond?
Again, it is more energy-efficient for chlorine to gain one electron than to lose seven. Therefore, it tends to gain an electron to create an ion with 17 protons, 17 neutrons, and 18 electrons, giving it a net negative (–1) charge. It is now referred to as a chloride ion.
Which of the following occurs when an ionic bond is formed?
Ionic bonding is the complete transfer of valence electron(s) between atoms. It is a type of chemical bond that generates two oppositely charged ions. In ionic bonds, the metal loses electrons to become a positively charged cation, whereas the nonmetal accepts those electrons to become a negatively charged anion.
How many electrons will sodium gain or lose when it forms an ion?
Tell students that when an atom gains or loses an electron, it becomes an ion. Sodium loses an electron, leaving it with 11 protons, but only 10 electrons. Since it has 1 more proton than electrons, sodium has a charge of +1, making it a positive ion.
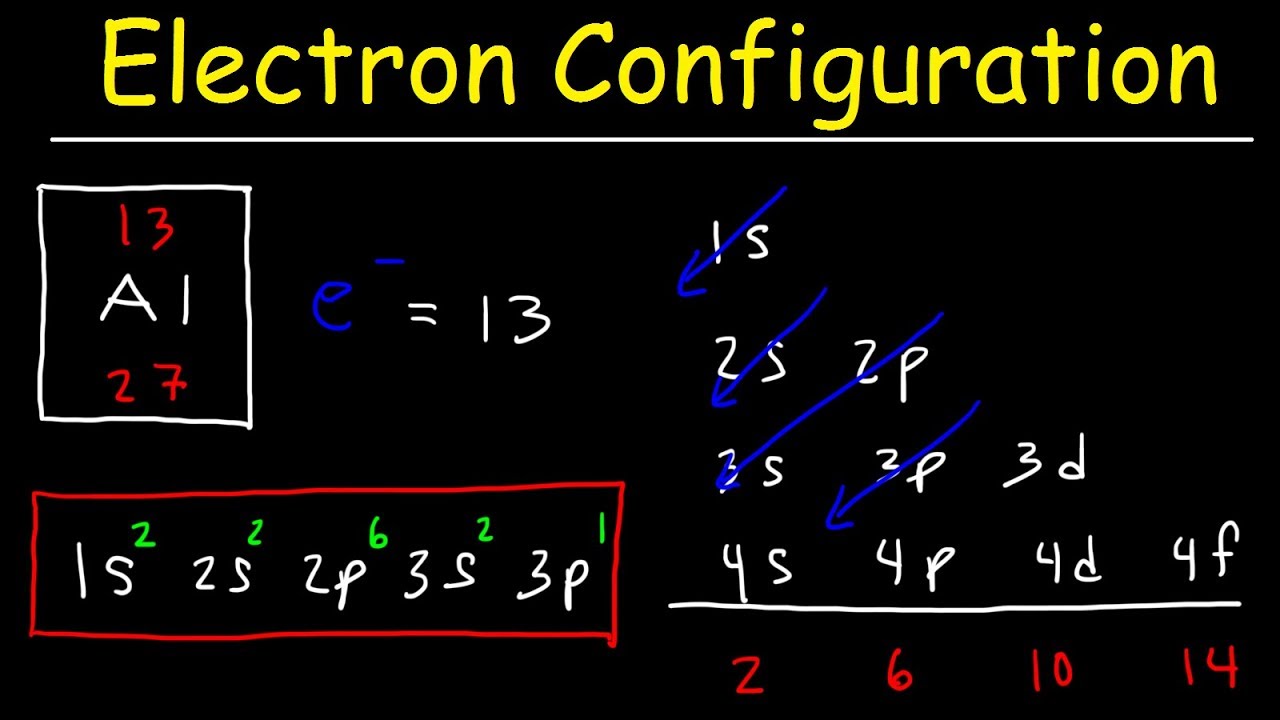
Electron Configuration – Basic introduction
Images related to the topicElectron Configuration – Basic introduction
When a chlorine atom gains one more electron to become stable What type of ion will it form?
Chlorine also has ten core electrons and valence electrons in the third energy level. However, chlorine has seven valence electrons, one less than the noble gas argon, which has eight valence electrons. Thus, chlorine will gain one electron, forming the anion, Cl–, and achieving a stable noble gas configuration.
How many electrons will chlorine gain or lose when it forms an ion quizlet?
How many electrons will chlorine gain or lose when it forms an ion? zero.
Related searches to When a sodium atom reacts with a chlorine atom to form a compound the electron configuration?
- what type of bond is formed between the two chlorine atoms in a chlorine molecule
- which is the correct electron-dot formula for a molecule of chlorine?
- when a sodium atom reacts with a chlorine atom to form a compound the electron configuration
- a barium atom attains a stable electron configuration when it bonds with
- which is the correct electron dot formula for a molecule of chlorine
- when two atoms form a chemical bond by sharing electrons
- which atom will form an ionic bond with a br atom
- as a chemical bond forms between two hydrogen atoms, the potential energy of the atoms
- which type of bonding is characteristic of a substance that has a high
- as a bond between a hydrogen atom and a sulfur atom is formed electrons are
- as a bond between a hydrogen atom and a sulfur atom is formed, electrons are
- which atom will form an ionic bond with a br atom?
- as a chemical bond forms between two hydrogen atoms the potential energy of the atoms
Information related to the topic When a sodium atom reacts with a chlorine atom to form a compound the electron configuration?
Here are the search results of the thread When a sodium atom reacts with a chlorine atom to form a compound the electron configuration? from Bing. You can read more if you want.
You have just come across an article on the topic When a sodium atom reacts with a chlorine atom to form a compound the electron configuration?. If you found this article useful, please share it. Thank you very much.
