Are you looking for an answer to the topic “When a sodium atom transfers an electron to a chlorine atom?“? We answer all your questions at the website Ecurrencythailand.com in category: +15 Marketing Blog Post Ideas And Topics For You. You will find the answer right below.
When a sodium atom transfers an electron to a chlorine atom the ions will separate in the presence of water. the sodium atom becomes a positively charged ion. the chlorine atom becomes a negatively charged ion. the positive and negative ions will attract each other, forming a crystal if no water is present.With dissociation, sodium loses one electron (loss of a negative charge) and becomes positive (sodium ion) (see Figure 2). Chlorine gains one electron and becomes negative (chloride ion).The protons of the two atoms attract the electrons of the other atom. The thicker arrow shows that chlorine has a stronger attraction for electrons than sodium has. During the interactions between the atoms, the electron in sodium’s outer energy level is transferred to the outer energy level of the chlorine atom.

What happens when a sodium ion and a chlorine ion exchange an electron?
With dissociation, sodium loses one electron (loss of a negative charge) and becomes positive (sodium ion) (see Figure 2). Chlorine gains one electron and becomes negative (chloride ion).
Why is an electron transferred from the sodium to chlorine?
The protons of the two atoms attract the electrons of the other atom. The thicker arrow shows that chlorine has a stronger attraction for electrons than sodium has. During the interactions between the atoms, the electron in sodium’s outer energy level is transferred to the outer energy level of the chlorine atom.
How Ionic Bonds Form (Basic)
Images related to the topicHow Ionic Bonds Form (Basic)

When one electron of sodium is transferred to chlorine What is the charge of chlorine?
The chlorine atom now has eight valence electrons in its third energy level, having gained the electron from the sodium atom. Since it gained one electron, it is now a chloride ion with a charge of 1− .
What kind of interaction occurs between atoms of sodium and chlorine?
Ionic bonds usually occur between metal and nonmetal ions. For example, sodium (Na), a metal, and chloride (Cl), a nonmetal, form an ionic bond to make NaCl.
Ionic Bond in Sodium Chloride NaCl Std 9 10
Images related to the topicIonic Bond in Sodium Chloride NaCl Std 9 10
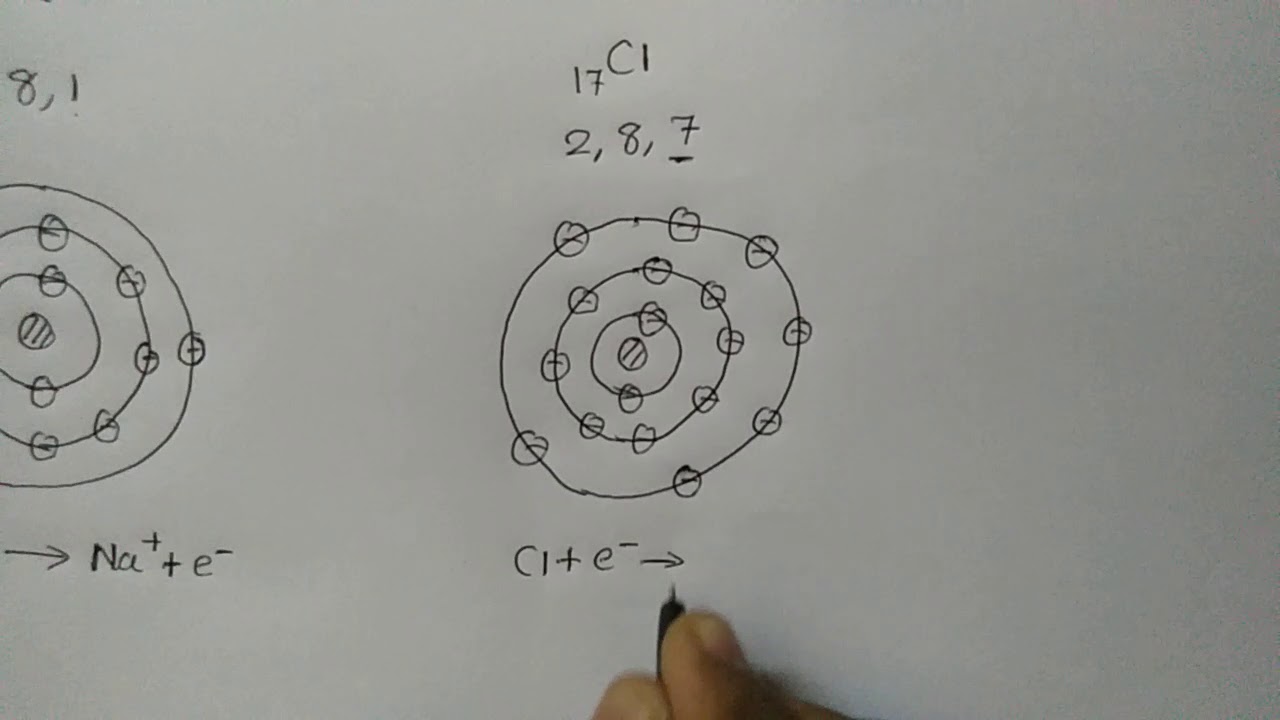
What do sodium and chlorine make when combined?
Sodium and chlorine, two highly reactive elements, combine to form the stable compound sodium chloride (ordinary table salt).
What happens when chlorine reacts with Na?
Sodium and chlorine react vigorously when heated, giving an orange flame and clouds of white sodium chloride.
See some more details on the topic When a sodium atom transfers an electron to a chlorine atom? here:
Biology 1: Chapter 2 Flashcards | Quizlet
Ionic bonds are formed when two atoms are held together by an attraction between ions. Sodium transfers a negatively charged electron over to chlorine.
Solved When a sodium atom transfers an electron to a – Chegg
Question: When a sodium atom transfers an electron to a chlorine atom the ions will separate in the presence of water. the sodium atom becomes a positively …
Ionic Bond – an overview | ScienceDirect Topics
Let’s examine the ionic bond in sodium chloride. A sodium atom, which has 11 protons and 11 electrons, has a single valence electron in its 3s subshell.
Sodium Chloride – Garden and Plate
When sodium and chlorine atoms come together to form sodium chloride (NaCl), they transfer an electron. The sodium (Na) atom transfers one electron to the …
What happens when sodium chloride forms?
Sodium chloride is formed when sodium atoms interact with chlorine atoms. When this occurs, sodium will donate an electron (which is a negatively-charged particle) to chlorine. This makes sodium slightly positive and chlorine slightly negative.
Atomic Structure of the Sodium Atom (Na)
Images related to the topicAtomic Structure of the Sodium Atom (Na)
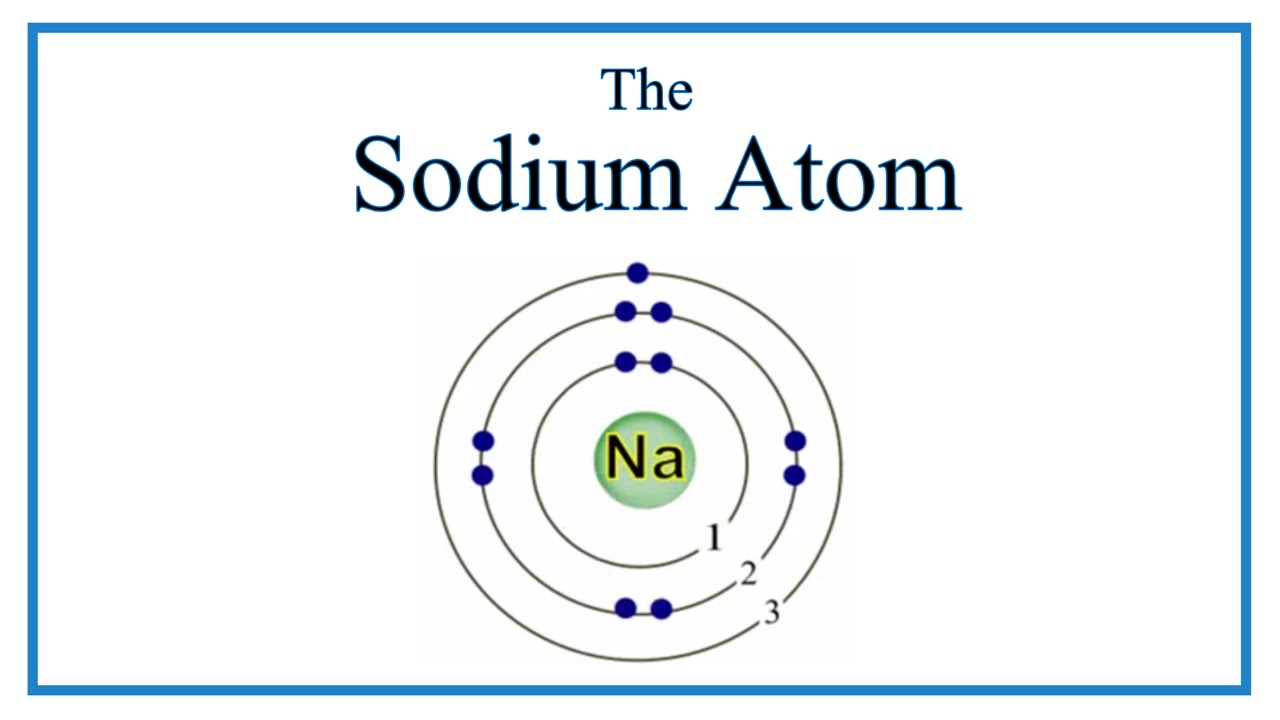
What is the charge of the sodium and the charge of chlorine?
It is the main component of table salt, used in cooking. Sodium chloride contains sodium ions, each with a +1 charge, and chloride ions, each with a -1 charge. Overall, the compound has no charge, because the positive sodiums balance out the charge on the negative chlorides, and vice versa.
What kind of interaction occurs between atoms of sodium and chlorine quizlet?
Table salt is an example of an ionic compound. Sodium and chlorine ions come together to form sodium chloride, or NaCl. The sodium atom in this compound loses an electron to become Na+, while the chlorine atom gains an electron to become Cl-.
Related searches to When a sodium atom transfers an electron to a chlorine atom?
- the subatomic particles that are found in the nucleus of an atom are the
- some insects can stride on the surface of water because water
- an ionic bond is formed when
- what happens when a sodium atom transfers an electron to a chlorine atom
- which of the following statements about basic solutions is true?
- an ionic bond is formed between
- a water molecule as shown here is polar because of
- which of these does not occur when a sodium atom transfers an electron to a chlorine atom
- which of these does not occur when a sodium atom transfers an electron to a chlorine atom?
- when a sodium atom transfers an electron to a chlorine atom they become
- when a sodium atom transfers an electron to a chlorine atom quizlet
- when a sodium atom transfers an electron to a chlorine atom
- which of the following statements about basic solutions is true
Information related to the topic When a sodium atom transfers an electron to a chlorine atom?
Here are the search results of the thread When a sodium atom transfers an electron to a chlorine atom? from Bing. You can read more if you want.
You have just come across an article on the topic When a sodium atom transfers an electron to a chlorine atom?. If you found this article useful, please share it. Thank you very much.