Are you looking for an answer to the topic “When a system loses energy the surroundings energy goes up?“? We answer all your questions at the website Ecurrencythailand.com in category: +15 Marketing Blog Post Ideas And Topics For You. You will find the answer right below.
Keep Reading

When a system loses energy Its energy goes up?
When a system loses energy, the surroundings energy goes UP. A combustion reaction, like a candle burning, loses energy and so the surroundings get hot. In a chemical reaction, the “system” is the chemicals involved in the reaction and the beaker that contains them. The surroundings is everything outside the beaker.
When the system releases energy to its surroundings?
A process in which heat (q) is transferred from a system to its surroundings is described as exothermic. By convention, q<0 for an exothermic reaction. When heat is transferred to a system from its surroundings, the process is endothermic.
First Law of Thermodynamics, Basic Introduction – Internal Energy, Heat and Work – Chemistry
Images related to the topicFirst Law of Thermodynamics, Basic Introduction – Internal Energy, Heat and Work – Chemistry

When energy is transferred from the surroundings to the system the universe gains energy?
One of the most important observations in science is that energy can be neither created nor destroyed: Energy is conserved. Any energy that is lost by the system must be gained by the surroundings, and vice versa.
When energy is transferred as heat from system to the surroundings the change in h is negative?
When energy is transferred as heat from the surroundings to the system, ΔH is negative. The evaporation of water is an exothermic process. A combustion reaction is exothermic. When energy is transferred as heat from the system to the surroundings, ΔH is negative.
What name is given to a reaction that gives out energy to the surroundings give the full name?
Exothermic reactions
These are reactions that transfer energy to the surroundings (ie the energy exits from the reaction, hence the name exothermic). The energy is usually transferred as heat energy, causing the reaction mixture and its surroundings to become hotter.
When the system does work on the surroundings?
When a system does work on the surroundings, the system’s internal energy decreases. When a system has work done on it, the internal energy of the system increases. Like heat, the energy change from work always occurs as part of a process: a system can do work, but doesn’t contain work.
Which process is endothermic?
An endothermic process is where heat is transferred from the surroundings to the systems. So the system has gained heat from the surroundings. The change in enthalpy, delta H is positive for an endothermic process. An example could be melting an ice cube.
See some more details on the topic When a system loses energy the surroundings energy goes up? here:
Heat and Work: TRUE or FALSE Flashcards | Quizlet
When a system loses energy, the surroundings energy goes UP. · A combustion reaction, like a candle burning, loses energy and so the surroundings get hot. · In a …
Exothermic & Endothermic Reactions – Energy Foundations …
In endothermic reactions, more energy is absorbed when the bonds in the reactants are broken than is released when new bonds are formed in the products.
The laws of thermodynamics (article) | Khan Academy
An open system can exchange both energy and matter with its surroundings. The stovetop example would be an open system, because heat and water vapor can be …
5.2 The First Law of Thermodynamics
When hydrogen and oxygen form water, the system loses energy to the surroundings as heat; because heat is lost from the system, the internal energy of the …
What event is endothermic?
Melting ice cubes. Melting solid salts. Evaporating liquid water. Converting frost to water vapor (melting, boiling, and evaporation, in general, are endothermic processes.
What happens when a system loses energy Choose the correct answer?
– If a system loses energy, the surroundings gain the exact same amount of energy, and vice versa. – Internal energy is the sum of the kinetic and potential energies of all the particles that make up a system; it is a state function.
When heat is absorbed by the system from the surroundings the process is said to be?
A chemical reaction or physical change is endothermic if heat is absorbed by the system from the surroundings. In the course of an endothermic process, the system gains heat from the surroundings and so the temperature of the surroundings decreases.
In which direction does energy flow into the system from the surroundings from the system into the surroundings?
In which direction does energy flow? a. If the reactants have a lower internal energy than the products, ΔEsystem is positive and energy flows into the system from the surroundings.
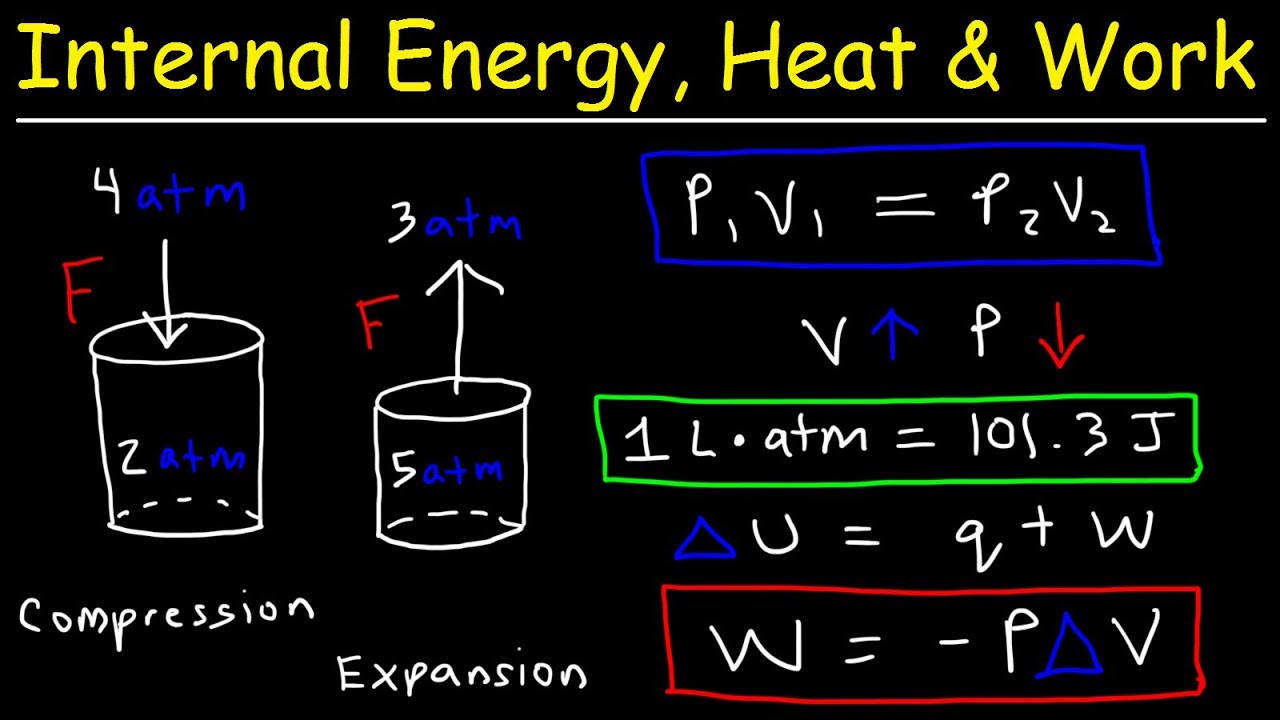
Internal Energy, Heat, and Work Thermodynamics, Pressure Volume, Chemistry Problems
Images related to the topicInternal Energy, Heat, and Work Thermodynamics, Pressure Volume, Chemistry Problems
Does endothermic gain or lose energy?
This is when a reaction starts colder and ends up hotter, taking in energy from start to finish. In an endothermic reaction, the system gains heat as the surroundings cool down. In an exothermic reaction, the system loses heat as the surroundings heat up.
Which type of energy transfers heat from the surroundings to the system?
Thermal radiation is a form of heat transfer because the electromagnetic radiation emitted from the source carries energy away from the source to surrounding (or distant) objects.
Why would an exothermic reaction transfer heat to the surroundings?
Exothermic reactionIn an exothermic reaction, the total energy of the products is less than the total energy of the reactants. Therefore, the change in enthalpy is negative, and heat is released to the surroundings.
Where does the energy absorbed from the surroundings go?
| Endothermic Reactions | Exothermic Reactions |
|---|---|
| Heat is absorbed from the surroundings; as a result, the surroundings get cold. | Heat is released by the reaction to surroundings; surroundings feel hot. |
| The reactants are lower in energy than the products | The products are lower in energy than the reactants |
What happens in an endothermic reaction?
An endothermic reaction is any chemical reaction that absorbs heat from its environment. The absorbed energy provides the activation energy for the reaction to occur. A hallmark of this type of reaction is that it feels cold.
Does exothermic release energy?
Chemical reactions that release energy are called exothermic. In exothermic reactions, more energy is released when the bonds are formed in the products than is used to break the bonds in the reactants. Exothermic reactions are accompanied by an increase in temperature of the reaction mixture.
What does endothermic mean in chemistry?
Definition of endothermic
1 : characterized by or formed with absorption of heat. 2 : warm-blooded.
What happens in an exothermic reaction?
An exothermic reaction is a chemical reaction that releases energy by light or heat. It is the opposite of an endothermic reaction.
Which is an exothermic reaction?
An exothermic reaction is a reaction in which energy is released in the form of light or heat. Thus in an exothermic reaction, energy is transferred into the surroundings rather than taking energy from the surroundings as in an endothermic reaction. In an exothermic reaction, change in enthalpy ( ΔH) will be negative.
Is work done on the surroundings positive or negative?
Work is done by the system on its surroundings, so work is negative.
#Virgo it maybe competitions rivals surrounding you but stand your ground.
Images related to the topic#Virgo it maybe competitions rivals surrounding you but stand your ground.

What is a surrounding in thermodynamics?
The space outside the thermodynamic system is known as the surroundings, a reservoir, or the environment. The properties of the walls determine what transfers can occur.
What happens to the internal energy of a system either increases or decreases if work is done a by the system B on the system?
(b) If work is done by the system, internal energy will decrease.
Related searches to When a system loses energy the surroundings energy goes up?
- when energy is transferred as heat from the surroundings to the system, δh is negative.
- a combustion reaction is exothermic
- h for an exothermic reaction is positive
- when energy is transferred as heat from the surroundings to the system h is negative
- the evaporation of water is an exothermic process.
- which of the reactions are exothermic
- which of the reactions are exothermic?
- the evaporation of water is an exothermic process
- h for an endothermic reaction is positive
- energy is released to the surroundings
- which of the following could be classified as the surroundings
- δh for an endothermic reaction is positive.
- a combustion reaction is exothermic.
- when a system loses energy the surroundings energy goes up or down
Information related to the topic When a system loses energy the surroundings energy goes up?
Here are the search results of the thread When a system loses energy the surroundings energy goes up? from Bing. You can read more if you want.
You have just come across an article on the topic When a system loses energy the surroundings energy goes up?. If you found this article useful, please share it. Thank you very much.